Update: Multistate Outbreak of Fungal Meningitis and Other Infections Associated with Contaminated Steroid Medication
Distributed via the CDC Health Alert Network
November 20, 2012, 15:00 ET (3:00 PM ET)
CDC HAN-00335-2012-11-20-ADV-N
Summary
The Centers for Disease Control and Prevention (CDC) continues to work closely with state public health departments on a multistate investigation of fungal meningitis and other infections among patients who received a methylprednisolone acetate (MPA) injection prepared by the New England Compounding Center (NECC) in Framingham, Mass. This HAN notice provides updated information on the following:
- Epidural abscess and other clinical syndromes being diagnosed in exposed patients
- Diagnostic and treatment recommendations for clinicians
Background
As of November 19, 2012, a total of 490 cases, which includes 34 deaths, have been reported in 19 states (see CDC’s website for up-to-date information about case count and distribution by state). Exserohilum rostratum continues to be the predominant fungus identified in patients and confirmed by the CDC laboratory.
Clinical Syndromes Reported to CDC
Currently, more than 7 weeks after the three implicated lots of MPA1 were recalled, CDC continues to receive reports of fungal infection in exposed patients. Previously, the majority of new cases reported to CDC were patients with fungal meningitis following injection.
Although cases of fungal meningitis continue to be reported, CDC has recently observed an increase in the number of patients presenting with evidence of epidural abscess, phlegmon, discitis, vertebral osteomyelitis, or arachnoiditis at or near the site of injection. These complications have occurred in patients with and without evidence of fungal meningitis.
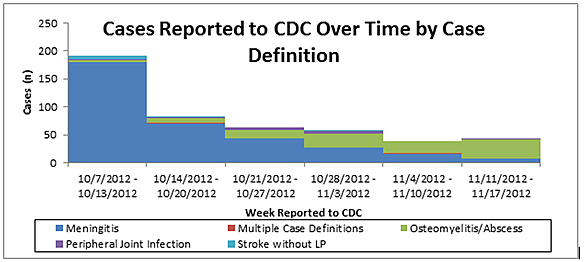
Of the 91 cases reported to CDC since November 4, 2012, a total of 26 (29%) were classified as meningitis, 61 (67%) had spinal or paraspinal epidural abscess or osteomyelitis, 2 (2%) had peripheral joint infection, and 2 (2%) had more than one condition(Figure 1).
Figure 1
Note: Data presented in Figure 1 are preliminary and subject to change. Additional patients may ultimately meet multiple case definitions (e.g., meningitis and osteomyelitis/abscess) as more time elapses and additional information is provided to CDC. Reporting dates to CDC may lag behind onset dates.
Diagnostic and Treatment Guidance
As a reminder, CDC’s current diagnostic and treatment guidance addresses management of patients with epidural abscess or other complications at or near the injection site. These localized infections may occur in isolation or in patients previously diagnosed with fungal meningitis. Although patients with these localized infections frequently have new or worsening back pain, symptoms may be mild or clinically difficult to distinguish from the patient’s baseline chronic pain. Based on current information, CDC recommends the following diagnostic protocol:
- In patients with new or worsening symptoms at or near the injection site, physicians should obtain an MRI with contrast of the symptomatic area(s), if not contraindicated. This recommendation also applies to patients being treated for meningitis. In some cases, radiologic evidence of abscess or phlegmon has become apparent on repeat MRI studies performed subsequent to an initially normal imaging procedure. Clinicians should therefore have a low threshold for repeat MRI studies in patients who continue to have symptoms localizing to the site of injection, even after a normal study. However, the optimal duration between MRI studies is unknown.
- CDC has received reports of patients being treated for fungal meningitis who had no previous evidence of localized infection at the site of injection, but who were subsequently found to have evidence of localized infection (e.g., epidural abscess, phlegmon, discitis, vertebral osteomyelitis, or arachnoiditis) on imaging studies. Therefore, in patients being treated for meningitis, even in the absence of new or worsening symptoms at or near the injection site, clinicians should strongly consider obtaining an MRI of the injection site approximately 2-3 weeks after diagnosis of meningitis. Early identification of new disease may facilitate additional specific interventions (e.g., drainage) and provide information for measuring effectiveness of therapy thereafter.
- For patients demonstrated to have epidural abscess, phlegmon, discitis, vertebral osteomyelitis, or arachnoiditis, early consultation with a neurosurgeon to discuss whether surgical management, including debridement, is warranted in addition to antifungal therapy (for information about antifungal therapy, see Interim Treatment Guidance for Central Nervous System and Parameningeal Infections Associated with Injection of Contaminated Steroid Products http://www.cdc.gov/hai/outbreaks/clinicians/guidance_cns.html).
CDC continues to gather data from existing and newly reported cases of infection and will use this information to inform updates to existing guidance. Healthcare professionals with patients under their care should check CDC’s website for the most up-to-date clinical guidance because information is subject to change.
1NECC lots of methylprednisolone acetate (PF) 80mg/ml:
Methylprednisolone Acetate (PF) 80 mg/ml Injection, Lot #05212012@68, BUD 11/17/2012
Methylprednisolone Acetate (PF) 80 mg/ml Injection, Lot #06292012@26, BUD 12/26/2012
Methylprednisolone Acetate (PF) 80 mg/ml Injection, Lot #08102012@51, BUD 2/6/2013
The Centers for Disease Control and Prevention (CDC) protects people’s health and safety by preventing and controlling diseases and injuries; enhances health decisions by providing credible information on critical health issues; and promotes healthy living through strong partnerships with local, national and international organizations.
Department of Health and Human Services
HAN Message Types
- Health Alert: Conveys the highest level of importance about a public health incident.
- Health Advisory: Provides important information about a public health incident.
- Health Update: Provides updated information about a public health incident.
###
This message was distributed to state and local health officers, state and local epidemiologists, state and local laboratory directors, public information officers, HAN coordinators, and clinician organizations.
###