Recommendations for Fully Vaccinated People
COVID-19 Homepage
Considerations for SARS-CoV-2 Antigen Testing for Healthcare Providers Testing Individuals in the Community
Summary of Recent Changes
Key Points
- This guidance is intended for healthcare providers who order antigen tests, receive antigen test results, or perform point-of-care antigen testing for SARS-CoV-2. It is also intended for laboratory and testing professionals and public health practitioners who perform antigen testing and reporting for SARS-CoV-2 in a laboratory setting or at the point of care.
- The guidance supports effective clinical use of antigen tests for different testing situations.
- This guidance focuses on the use of antigen tests to diagnose new infections.
- Guidance for performing antigen self-tests (also known as at-home tests) can be found on CDC’s Self-Testing webpage.
Antigen Testing for SARS-CoV-2
General Guidance
Antigen tests are immunoassays that detect the presence of a specific viral antigen, which suggests current viral infection. Antigen tests are commonly used in the diagnosis of respiratory pathogens, including influenza viruses and respiratory syncytial virus (RSV). The U.S. Food and Drug Administration (FDA) has granted emergency use authorization (EUA) for antigen tests that can identify SARS-CoV-2. See FDA’s list of In Vitro Diagnostics EUAs.
SARS-CoV-2 antigen tests are currently authorized for nasopharyngeal swab and nasal swab specimens. The currently authorized antigen tests include point-of-care (POC), laboratory-based, and self-tests available without a prescription. Certain tests have age limitations; refer to FDA’s website for more details.
Antigen tests produce results quickly (within minutes), and most can be used at the POC or at home. Most self-tests, or at-home tests, are antigen tests. Antigen tests are better at detecting a SARS-CoV-2 infection when someone has COVID-19 symptoms compared to if they do not. Antigen tests for SARS-CoV-2 are generally less sensitive than real-time reverse transcription polymerase chain reaction (RT-PCR) and other nucleic acid amplification tests (NAATs), which detect and amplify the presence of viral nucleic acid. For this reason, in situations where test sensitivity is of paramount importance, NAAT tests are preferred. For additional comparison between NAAT and antigen tests, please see the Summary Table of NAAT and Antigen Test Differences.
Accurate identification of infection and clinical management of COVID-19 requires performing the test properly and correctly interpreting the results. Patients who test positive should follow CDC isolation guidance for next steps. All initial negative antigen test results should be confirmed with a NAAT or repeated with additional antigen tests following FDA’s recommendations on repeat testing.
Performance of Antigen Tests for SARS-CoV-2
It is important for healthcare providers and testing professionals to understand the performance characteristics, including sensitivity, specificity, and positive and negative predictive values, of the antigen test being used, and to follow the manufacturer’s instructions for use, which summarize performance characteristics. See FDA’s In Vitro Diagnostics EUA for detailed information about specific authorized tests.
The “gold standard” for clinical diagnostic detection of SARS-CoV-2 remains laboratory-based (moderate- and high-complexity) NAATs. In situations where test sensitivity is of paramount importance, NAAT tests are preferred. Antigen tests do not have the same limits of detection as most NAATs, which have a higher sensitivity. Compared to NAATs, antigen tests are more likely to return a false negative, especially when testing before symptom onset when the level of antigens in a specimen is lower.
The specificity of antigen tests is comparable to NAATs, which means that false positive test results are unlikely when an antigen test is used according to the manufacturer’s instructions. Despite the high specificity of antigen tests, false positive results can occur, especially when used in situations where the pretest probability is low – a circumstance that is true for all in vitro diagnostic tests.
Positive and negative predictive values of all in vitro diagnostic tests (e.g., NAAT and antigen tests) vary depending upon the pretest probability. Pretest probability is the likelihood that the person being tested actually has the infection. Clinicians should consider the following when determining pretest probability for COVID-19:
- If the person has symptoms
- If the person was exposed to COVID-19
- If the prevalence of COVID-19 in the community is high
- If the person does not have symptoms
- If the person was not exposed to COVID-19
- If the prevalence of COVID-19 in the community is low
Public health departments may publish COVID-19 data on case rates or test positivity for their communities.
Interpreting the Results of Antigen Testing for SARS-CoV-2
When testing a person in a community setting, generally, healthcare providers can rely upon a positive antigen test result for a patient because the specificity of current FDA-authorized antigen tests is high. Patients who test positive should follow CDC isolation guidance.
The sensitivity of current FDA-authorized antigen tests varies, but is lower than NAATs. A negative test result means the test did not detect the virus, but this does not rule out an infection. Negative antigen test results should be considered “presumptive,” meaning that they are preliminary results; therefore, an initial negative antigen test result should always be followed by additional testing. If the additional testing includes NAAT, and the results are discordant, the NAAT result should be interpreted as definitive for the purposes of clinical diagnosis. If repeating using antigen tests, follow FDA guidance on repeat testing.
A person who has received a negative antigen test result and then a positive repeat test should follow CDC’s guidance for isolation from the date of symptom onset (if individual had symptoms) or the date of the repeat test (if no symptoms).
A symptomatic person who has received a negative COVID-19 test result should consider diagnostic testing for other pathogens that can cause acute febrile respiratory illness, such as influenza and RSV, and take everyday preventive actions to prevent spreading an illness to others.
CDC has developed an algorithm for healthcare providers to advise on antigen testing in a community setting. See Figure 1, also available as a PDF.
Figure 1. Recommendations to Healthcare Providers on Interpreting Antigen Test Results for Diagnostic Purposes
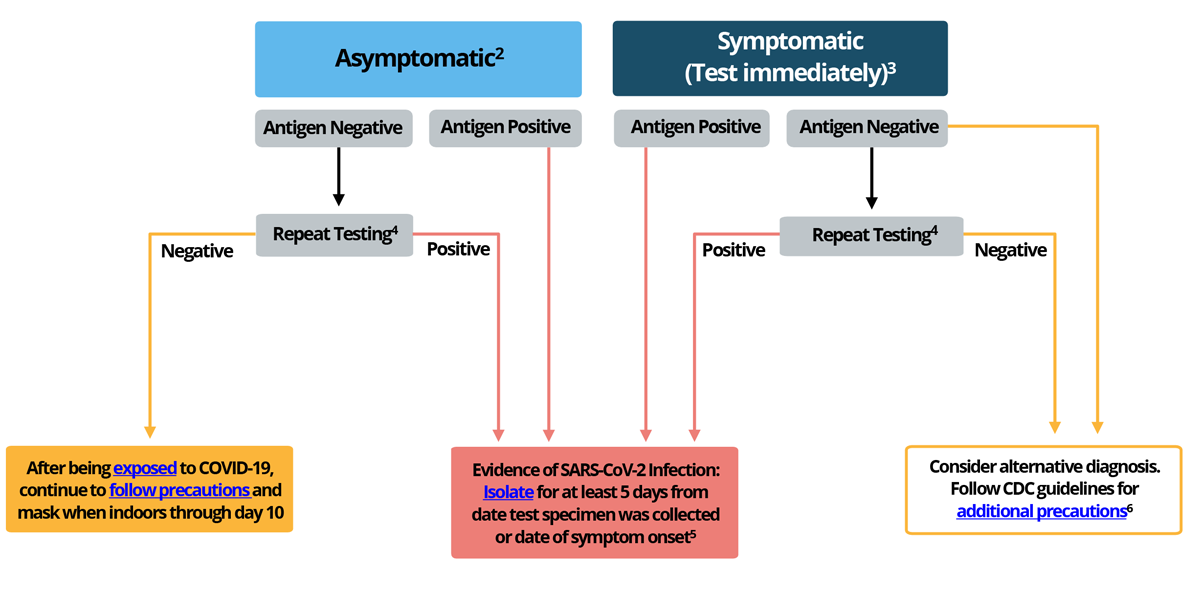
1This guidance does not apply to specific settings (e.g. congregate, high-risk, and inpatient healthcare settings).
2For those who are traveling: follow guidance for domestic and international travel during the COVID-19 pandemic. Take precautions while traveling. People in certain high-risk settings may need to test as part of a screening testing program.
3Symptomatic individuals should take general public health precautions to prevent spreading an illness to others.
4 In situations where test sensitivity is of paramount importance, NAAT testing should take place as soon as possible, and not longer than 48 hours after the initial antigen testing. If the results are discordant, the NAAT result should be interpreted as definitive. If using another antigen test, follow FDA guidance on repeat testing.
5 See CDC’s guidance on treatments for COVID-19, particularly if individual is at high-risk of severe disease from COVID-19. Also see CDC’s guidance on Isolation and Exposure to COVID-19.
6 Early diagnosis and treatment are important in preventing severe illness for many pathogens that cause acute febrile respiratory diseases; additional diagnostic testing should be pursued in conjunction with repeat/confirmatory testing for COVID-19.
For additional details on testing recommendations, see CDC’s Overview of Testing for SARS-CoV-2.
Confirmatory Testing When Using Antigen Tests for SARS-CoV-2
In situations where test sensitivity is of paramount importance, providers may choose to confirm a negative antigen test result with a laboratory-based NAAT, especially if the result of the antigen test is inconsistent with the clinical context. Confirmatory testing should take place as soon as possible after the antigen test, and not longer than 48 hours after the initial antigen testing. If the results are discordant between the antigen test and the confirmatory NAAT, in general the confirmatory test result should be interpreted as definitive for the purpose of clinical diagnosis. Based on their instructions for use, NAATs that generate presumptive results should not be used for confirmatory testing.
For confirmatory testing, CDC recommends using an FDA-authorized laboratory-based NAAT. See FDA’s Molecular Diagnostic Tests for SARS-CoV-2. CDC does not recommend NAATs that use oral specimens (e.g., saliva) for confirmatory testing and instead suggests the use of specimens that are considered optimal for detection, such as nasopharyngeal, nasal mid-turbinate, and anterior nasal swabs. See CDC’s guidance for Nucleic Acid Amplification Tests (NAATs).
Processing of Antigen Tests for SARS-CoV-2
The Conditions of Authorization in the antigen EUAs specify that CLIA-certified laboratories and testing sites are to follow the manufacturer’s instructions for use, typically found in the package insert, when performing the test and reading test results. The authorized instructions for use for each test, including when and how to read each test, can also be found at FDA’s In Vitro Diagnostics EUA. Also see FDA’s At-Home COVID-19 Diagnostic Tests: Frequently Asked Questions. All testing for SARS-CoV-2, including antigen testing, depends on the integrity of the specimen, which is affected by procedures for both specimen collection and handling. See CDC’s Interim Guidelines for Collecting and Handling of Clinical Specimens for COVID-19 Testing.
Quality assurance procedures should be followed to prevent cross-contamination and inaccurate test results. For more information on proper specimen processing and handling for COVID-19 testing, including point-of-care tests, see CDC’s guidance on Point-of-Care Testing, and Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with Coronavirus Disease 2019 (COVID-19).
Reporting Antigen Test Results for SARS-CoV-2 to Health Departments and Patients
A CLIA-certified laboratory or testing site should review state-specific reporting requirements for COVID-19 testing. Antigen test results that are reported to public health departments must be clearly distinguished from other COVID-19 tests, such as NAATs and antibody tests.
For more information on how to report COVID-19 antigen test results, see CDC’s guidance on How to Report COVID-19 Laboratory Data which specifies what additional data should be collected and electronically reported to health departments along with COVID-19 diagnostic or screening test results. When possible, laboratory and testing professionals should collect and report complete patient demographic information and ensure that they report antigen test results using the proper LOINC code for their particular FDA-authorized tests. Facilities should refer to CDC’s LOINC In Vitro Diagnostic (LIVD) Test Code Mapping for SARS-CoV-2 Tests.
For long-term care facilities that are enrolled in CDC’s National Healthcare Safety Network (NHSN), the preferred method for reporting point-of-care SARS-CoV-2 testing data, including positive antigen test results, is through the NHSN.
Regulatory Requirements for Using Antigen Tests for SARS-CoV-2
FDA regulates in vitro diagnostic devices and has provided recommendations and information regarding EUA requests for COVID-19 diagnostic tests in the Policy for Coronavirus Disease-2019 Tests During the Public Health Emergency (Revised) (“Policy for COVID-19 Tests”) and the EUA templates referenced in that policy. COVID-19 tests and test systems used for diagnostic or screening testing, including those for antigen testing, must have received an EUA from FDA or be offered under the policies in FDA’s Policy for COVID-19 Tests. Every antigen test for SARS-CoV-2 authorized for use by FDA is included on FDA’s list of In Vitro Diagnostics EUAs. The intended use of each test, available in the Instructions for Use and in the Letter of Authorization, defines the population in which the test is intended to be used, the acceptable specimen types, and how the results should be used.
Laboratory and testing professionals who conduct diagnostic (including screening) testing for SARS-CoV-2 with antigen tests must also comply with Clinical Laboratory Improvement Amendments (CLIA). Those that intend to report patient-specific test results to a person or healthcare provider must first obtain a CLIA certificate and meet all requirements to perform that testing. For more information, see CMS’ How to Obtain a CLIA Certificate.