Summary* Recommendations: Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—(ACIP)—United States, 2013-14
Influenza Prevention and Control Recommendations
- Primary Changes and Updates in the Recommendations
- Timing of Vaccination
- Available Vaccine Products and Indications
- Persons At Risk for Medical Complications Due to Influenza
- Persons Who Live With or Care for Persons at Higher Risk for Influenza-Related Complications
- Vaccine Dose Considerations for Children 6 Months through 8 Years of Age
- Influenza Vaccination for Pregnant Women
- Influenza Vaccination of Persons with a History of Egg Allergy
- Influenza Vaccines and Use of Influenza Antiviral Medications
- Concurrent Administration of Influenza Vaccine With Other Vaccines
- Table 1. Influenza Vaccines — United States, 2013–14 Influenza Season
This document is a summary of the recommendations of the Advisory Committee on Immunization Practices for the 2013-2014 season in the United States. The full recommendations will be published in Morbidity and Mortality Weekly Report (MMWR).
Note on abbreviations: This document includes revised abbreviations to refer to currently available influenza vaccines. Specifically:
- The former abbreviation TIV (Trivalent Inactivated Influenza Vaccine, previously used for inactivated influenza vaccines) has been replaced with the new abbreviation IIV (Inactivated Influenza Vaccine). For 2013-14, IIVs as a class will include:
- egg-based and cell culture-based trivalent inactivated influenza vaccines (IIV3), and
- egg-based quadrivalent inactivated influenza vaccine (IIV4).
- RIV refers to recombinant hemagglutinin influenza vaccine, available as a trivalent formulation (RIV3) for 2013-14;
- LAIV refers to live-attenuated influenza vaccine, available as a quadrivalent formulation (LAIV4) for 2013-14.
- LAIV, IIV, and RIV denote vaccine categories; numeric suffix specifies the number of antigens in the vaccine.
- Where necessary to refer specifically to cell culture-based vaccine, the prefix “cc” is used (e.g., “ccIIV3”).
Primary Changes and Updates in the Recommendations
- Routine annual influenza vaccination of all persons aged 6 months and older continues to be recommended.
- 2013-14 U.S. trivalent influenza vaccines will contain an A/California/7/2009 (H1N1)-like virus, an H3N2 virus antigenically like the cell-propagated prototype virus A/Victoria/361/2011, and a B/Massachusetts/2/2012-like virus. Quadrivalent vaccines will include an additional vaccine virus, a B/Brisbane/60/2008-like virus.
- Several new, recently-licensed vaccines will be available for the 2013-14 season, and are acceptable alternatives to other licensed vaccines indicated for their respective age groups when otherwise appropriate:
- A quadrivalent live attenuated influenza vaccine (LAIV4; Flumist® Quadrivalent [MedImmune]) is expected to replace the trivalent (LAIV3) formulation. FluMist® Quadrivalent is indicated for healthy, nonpregnant persons aged 2 through 49 years;
- A quadrivalent inactivated influenza vaccine (IIV4; Fluarix® Quadrivalent [GlaxoSmithKline]) will be available, in addition to the previous trivalent formulation. Fluarix® Quadrivalent is indicated for persons aged 3 years and older;
- A quadrivalent inactivated influenza vaccine (IIV4; Fluzone® Quadrivalent [Sanofi Pasteur]) will be available in addition to the previous trivalent formulation. Fluzone® Quadrivalent is indicated for persons aged 6 months and older;
- A trivalent cell culture-based inactivated influenza vaccine (ccIIV3; Flucelvax® [Novartis]), which is indicated for persons aged 18 years and older; and
- A recombinant hemagglutinin (HA) vaccine (RIV3; FluBlok® [Protein Sciences]), which is indicated for persons aged 18 through 49 years.
- Within approved indications and recommendations, no preferential recommendation is made for any type or brand of licensed influenza vaccine over another.
Timing of Vaccination
- In general, health-care providers should begin offering vaccination soon after vaccine becomes available, and if possible, by October.
- All children aged 6 months–8 years who are recommended for 2 doses (Figure 1) should receive their first dose as soon as possible after vaccine becomes available; these children should receive the second dose ≥4 weeks later.
Available Vaccine Products and Indications
A variety of influenza vaccine products are available (Table 1), including (as of July 2013) five newly approved vaccines. For many vaccine recipients, more than one type or brand of vaccine may be appropriate within indications and ACIP recommendations. Where more than one type of vaccine is appropriate and available, no preferential recommendation is made for use of any influenza vaccine product over another.
Persons at Risk for Medical Complications Due to Influenza
Vaccination to prevent influenza is particularly important for persons who are at increased risk for severe complications from influenza, or at higher risk for influenza-related outpatient, emergency department, or hospital visits. When vaccine supply is limited, vaccination efforts should focus on delivering vaccination to the following persons (no hierarchy is implied by order of listing):
- All children aged 6 through 59 months;
- All persons aged ≥50 years;
- Adults and children who have chronic pulmonary (including asthma) or cardiovascular (except isolated hypertension), renal, hepatic, neurological, hematologic, or metabolic disorders (including diabetes mellitus);
- Persons who have immunosuppression (including immunosuppression caused by medications or by HIV infection);
- Women who are or will be pregnant during the influenza season;
- Children and adolescents (aged 6 months–18 years) who are receiving long-term aspirin therapy and who might be at risk for experiencing Reye’s syndrome after influenza virus infection;
- Residents of nursing homes and other long-term care facilities;
- American Indians/Alaska Natives;
- Persons who are morbidly obese (BMI ≥40).
Persons Who Live With or Care for Persons at Higher Risk for Influenza-Related Complications
All persons aged ≥6 months should be vaccinated annually. Continued emphasis should be placed on vaccination of persons who live with or care for persons at higher risk for influenza-related complications. When vaccine supply is limited, vaccination efforts should focus on delivering vaccination to persons at higher risk for influenza-related complications listed above, as well as these persons:
- Healthcare personnel (HCP);
- Household contacts (including children) and caregivers of children aged ≤59 months (i.e., aged <5 years) and adults aged ≥50 years, with particular emphasis on vaccinating contacts of children aged <6 months; and
- Household contacts (including children) and caregivers of persons with medical conditions that put them at higher risk for severe complications from influenza.
HCP and persons who are contacts of persons in these groups and who are not contacts of severely immunocompromised persons (those living in a protective environment) may receive any influenza vaccine which is otherwise indicated. Individuals who care for the severely immunocompromised should receive either IIV or RIV3.
Vaccine Dose Considerations for Children 6 Months through 8 Years of Age
Children aged 6 months through 8 years who are receiving influenza vaccine for the first time, and some in this age group who have previously been vaccinated, require two doses of vaccine administered ≥4 weeks apart. Two approaches for determining the number of doses are recommended, both of which are acceptable:
- The first approach, outlined in the flowchart (Figure 1), takes into consideration only doses of seasonal influenza vaccine received since July 1, 2010. This approach has the advantage of simplicity, particularly in settings in which it is difficult to ascertain vaccination history prior to the 2010-11 season. Using this approach, children 6 months through 8 years of age need only 1 dose of vaccine in 2013-14 if they received a total of 2 or more doses of seasonal vaccine since July 1, 2010. Children who did not receive a total of 2 or more doses of seasonal vaccine since July 1, 2010 require 2 doses in 2013-14.
- In settings where adequate vaccination history from prior to the 2010-11 season is available, the second approach may be used. By this approach (Figure 1, footnote), if a child 6 months through 8 years of age is known to have received at least 2 doses of seasonal influenza vaccine during any prior season, and at least 1 dose of a 2009(H1N1)-containing vaccine–i.e., 2010-11, 2011-12, or 2012-13 seasonal vaccine or the monovalent 2009(H1N1) vaccine–then the child needs only 1 dose for 2013-14. Using this approach, children 6 months through 8 years of age need only 1 dose of vaccine in 2013-14 if they have received any of the following:
- 2 or more doses of seasonal influenza vaccine since July 1, 2010 or;
- 2 or more doses of seasonal influenza vaccine before July 1, 1010 and 1 or more doses of monovalent 2009(H1N1) vaccine or;
- 1 or more doses of seasonal influenza vaccine before July 1, 2010 and 1 or more doses of seasonal influenza vaccine since July 1, 2010
Children 6 months through 8 years of age for whom one of these conditions is not met require 2 doses in 2013-14.
Influenza Vaccination for Pregnant Women
- Women who are or will be pregnant during influenza season should receive IIV. Live attenuated influenza vaccine (LAIV) is not recommended for use during pregnancy.
- Postpartum women can receive either LAIV or IIV.
- Pregnant and postpartum women do not need to avoid contact with persons recently vaccinated with LAIV.
Influenza Vaccination of Persons with a History of Egg Allergy
- Persons with a history of egg allergy who have experienced only hives after exposure to egg should receive influenza vaccine. Because relatively little data are available for use of LAIV in this setting, IIV or RIV should be used. RIV is egg-free and may be used for persons aged 18-49 years who have no other contraindications. However, IIV (egg- or cell-culture based) may also be used, with the following additional safety measures (Figure 2):
- Vaccine should be administered by a healthcare provider who is familiar with the potential manifestations of egg allergy; and
- Vaccine recipients should be observed for at least 30 minutes for signs of a reaction after administration of each vaccine dose (1).
- Persons who report having had reactions to egg involving such symptoms as angioedema, respiratory distress, lightheadedness, or recurrent emesis; or who required epinephrine or another emergency medical intervention may receive RIV3, if aged 18 through 49 years and there are no other contraindications. If RIV3 is not available or the the receipient is not within the indicated age range, such persons should be referred to a physician with expertise in the management of allergic conditions for further risk assessment before receipt of vaccine (Figure 2).
- All vaccines should be administered in settings in which personnel and equipment for rapid recognition and treatment of anaphylaxis are available.
- Some persons who report allergy to egg might not be egg-allergic. Those who are able to eat lightly cooked egg (e.g., scrambled egg) without reaction are unlikely to be allergic. Egg-allergic persons might tolerate egg in baked products (e.g., bread or cake). Tolerance to egg-containing foods does not exclude the possibility of egg allergy (2). Egg allergy can be confirmed by a consistent medical history of adverse reactions to eggs and egg-containing foods, plus skin and/or blood testing for immunoglobulin E antibodies to egg proteins.
- For individuals who have no known history of exposure to egg, but who are suspected of being egg-allergic on the basis of previously performed allergy testing, consultation with a physician with expertise in the management of allergic conditions should be obtained prior to vaccination (Figure 2). Alternatively, RIV3 may be administered if the recipient is aged 18 through 49 years.
- A previous severe allergic reaction to influenza vaccine, regardless of the component suspected to be responsible for the reaction, is a contraindication to future receipt of the vaccine.
People with egg allergies can receive any licensed, recommended age-appropriate influenza (flu) vaccine (IIV4, RIV4, ccIIV4, or LAIV4) that is otherwise appropriate. People who have a history of severe egg allergy (those who have had any symptom other than hives after exposure to egg) should be vaccinated in a medical setting, supervised by a health care provider who is able to recognize and manage severe allergic reactions. Two completely egg-free flu vaccine options are available: Flublok Quadrivalent recombinant flu vaccine and Flucelvax Quadrivalent cell-based flu shot.
Influenza Vaccines and Use of Influenza Antiviral Medications
- Administration of IIV to persons receiving influenza antiviral drugs for treatment or chemoprophylaxis is acceptable.
- LAIV should not be administered until 48 hours after cessation of influenza antiviral therapy.
- If influenza antiviral medications are administered within 2 weeks after receipt of LAIV, the vaccine dose should be repeated 48 or more hours after the last dose of antiviral medication.
- Persons receiving antiviral drugs within the period 2 days before to 14 days after vaccination with LAIV should be revaccinated at a later date with any approved vaccine formulation (3).
Concurrent Administration of Influenza Vaccine With Other Vaccines
- Inactivated vaccines do not interfere with the immune response to other inactivated vaccines or to live vaccines.
- Inactivated or live vaccines can be administered simultaneously with LAIV.
- However, after administration of a live vaccine, at least 4 weeks should pass before another live vaccine is administered.
TABLE 1. Influenza Vaccines — United States, 2013–14 Influenza Season* |
||||||||
|---|---|---|---|---|---|---|---|---|
| Vaccine | Trade name | Manufacturer | Presentation | Mercury content (μg Hg/0.5 mL) | Ovalbulmin content (μg/0.5 mL) | Age indications | Route | |
| Inactivated Influenza Vaccine, Trivalent (IIV3), Standard Dose | Afluria® | CSL Limited | 0.5 mL single-dose prefilled syringe | 0.0 | ≤ 1 | ≥9 yrs.††† | IM† | |
| 5.0 mL multi-dose vial | 24.5 | ≤ 1 | ||||||
| Fluarix® | GlaxoSmithKline | 0.5 mL single-dose prefilled syringe | 0.0 | ≤0.05 | ≥3 yrs. | IM† | ||
| Flucelvax® | Novartis Vaccines | 0.5 mL single-dose prefilled syringe | 0.0 | §§§ | ≥18 yrs. | IM† | ||
| FluLaval® | ID Biomedical Corporation of Quebec (distributed by GlaxoSmithKline) | 5.0 mL multi-dose vial | <25.0 | ≤0.3 | ≥3 yrs | IM† | ||
| Fluvirin® | Novartis Vaccines | 0.5 mL single-dose prefilled syringe | ≤1 | ≤1 | ≥4 yrs. | IM† | ||
| 5.0 mL multi-dose vial | 25.0 | ≤1 | ||||||
| Fluzone® | Sanofi Pasteur | 0.25 mL single-dose prefilled syringe | 0.0 | **** | 6-35 mos. | IM† | ||
| 0.5 mL single-dose prefilled syringe | 0.0 | **** | ≥36 mos. | IM† | ||||
| 0.5 mL single-dose vial | 0.0 | **** | ≥36 mos. | IM† | ||||
| 5.0 mL multi-dose vial | 25.0 | **** | ≥6 mos. | IM† | ||||
| Fluzone® Intradermal†† | Sanofi Pasteur | 0.1 mL prefilled microinjection system | 0.0 | **** | 18-64 yrs. | ID§ | ||
| Inactivated Influenza Vaccine, Trivalent (IIV3), High Dose** | Fluzone® High-Dose | Sanofi Pasteur | 0.5 mL single-dose prefilled syringe | 0.0 | **** | ≥65 yrs. | IM† | |
| Inactivated Influenza Vaccine, Quadrivalent (IIV4), Standard Dose | Fluarix® Quadrivalent | GlaxoSmithKline | 0.5 mL single-dose prefilled syringe | 0.0 | ≤0.05 | ≥3 yrs. | IM† | |
| FluLaval® Quadrivalent | ID Biomedical Corporation of Quebec (distributed by GlaxoSmithKline) | 5.0 mL multi-dose vial | <25.0 | ≤0.03 | ≥3 yrs. | IM† | ||
| Fluzone® Quadrivalent | Sanofi Pasteur | 0.25 mL single-dose prefilled syringe | 0.0 | **** | 6-35 mos. | IM† | ||
| 0.5 mL single-dose prefilled syringe | 0.0 | **** | ≥36 mos. | IM† | ||||
| 0.5 mL single-dose vial | 0.0 | **** | ≥36 mos. | IM† | ||||
| Recombinant Influenza Vaccine, Trivalent (RIV3) | FluBlok® | Protein Sciences | 0.5 mL single-dose vial | 0.0 | 0.0 | 18-49 yrs. | IM† | |
| Live-attenuated Influenza Vaccine, Quadrivalent (LAIV4) | FluMist® Quadrivalent§§ | MedImmune | 0.2 mL prefilled intranasal sprayer | 0.0 (per 0.2 mL) | <0.24 (per 0.2 mL) | 2-49 yrs.*** | IN | |
| IIV=Inactivated Influenza Vaccine; IIV3=Inactivated Influenza Vaccine, Trivalent; IIV4=Inactivated Influenza Vaccine, Quadrivalent; RIV=Recombinant Influenza Vaccine LAIV=Live-Attenuated Influenza Vaccine; IM=intramuscular; ID=intradermal; IN=intranasal.
* Immunization providers should check Food and Drug Administration–approved prescribing information for 2013–14 influenza vaccines for the most complete and updated information, including (but not limited to) indications, contraindications, and precautions. Package inserts for US-licensed vaccines are available at http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm093833.htm. † For adults and older children, the recommended site of vaccination is the deltoid muscle. The preferred site for infants and young children is the anterolateral aspect of the thigh. Specific guidance regarding site and needle length for intramuscular administration may be found in the ACIP General Recommendations on Immunization [4]. § The preferred site is over the deltoid muscle. Fluzone® Intradermal is administered using the delivery system included with the vaccine. ** Inactivated influenza vaccine, high-dose: A 0.5-mL dose contains 60 μg of each vaccine antigen (180 μg total). †† Inactivated influenza vaccine, intradermal: A 0.1-mL dose contains 9 μg of each vaccine antigen (27 μg total). §§ It is anticipated that the quadrivalent formulation of FluMist® will replace the trivalent formulation for the 2013-14 season. FluMist® is shipped refrigerated and stored in the refrigerator at 35°F–46°F (2°C–8°C) after arrival in the vaccination clinic. The dose is 0.2 mL divided equally between each nostril. Health-care providers should consult the medical record, when available, to identify children aged 2–4 years with asthma or recurrent wheezing that might indicate asthma. In addition, to identify children who might be at greater risk for asthma and possibly at increased risk for wheezing after receiving LAIV, parents or caregivers of children aged 2–4 years should be asked: “In the past 12 months, has a health-care provider ever told you that your child had wheezing or asthma?” Children whose parents or caregivers answer “yes” to this question and children who have asthma or who had a wheezing episode noted in the medical record within the past 12 months should not receive FluMist®. *** Flumist® is indicated for healthy, non-pregnant persons aged 2-49 years. Individuals who care for severely immunosuppressed persons who require a protective environment should not receive FluMist given the theoretical risk of transmission of the live attenuated vaccine virus. ††† Age indication per package insert is ≥5 years; however, the ACIP recommends Afluria® not be used in children aged 6 months through 8 years because of increased risk of febrile reactions noted in this age group with CSL’s 2010 Southern Hemisphere IIV3. If no other age-appropriate, licensed inactivated seasonal influenza vaccine is available for a child aged 5–8 years who has a medical condition that increases the child’s risk for influenza complications, Afluria® can be used; however, providers should discuss with the parents or caregivers the benefits and risks of influenza vaccination with Afluria® before administering this vaccine. Afluria® may be used in persons aged ≥9 years (5). §§§ Information not included in package insert. The total egg protein is estimated to be less than 50 femtograms (5×10-14 grams) total egg protein, of which a fraction is ovalbumin, per 0.5 mL dose of Flucelvax®. **** Available upon request from upon request from Sanofi Pasteur, by telephone, 1-800-822-2463, or e-mail, MIS.Emails@sanofipasteur.com. |
||||||||
TABLE 2. Contraindications and Precautions to the Use of Influenza Vaccines, 2013-14.* |
||
|---|---|---|
| Vaccine | Contraindications | Precautions |
| IIV (includes IIV3, II4, and ccIIV) | History of severe allergic reaction to any component of the vaccine, including egg protein, or after previous dose of any influenza vaccine. | Moderate to severe illness with or without fever. History of Guillain-Barré syndrome within 6 weeks of receipt of influenza vaccine. |
| RIV | History of severe allergic reaction to any component of the vaccine. | Moderate to severe illness with or without fever. History of Guillain-Barré syndrome within 6 weeks of receipt of influenza vaccine. |
| LAIV | History of severe allergic reaction to any component of the vaccine, including egg protein, gentamicin, gelatin, and arginine, or after a previous dose of any influenza vaccine; Concomitant Aspirin therapy in children and adolescents. In addition, ACIP recommends against use in the following:
|
Moderate to severe illness with or without fever. History of Guillain-Barré syndrome within 6 weeks of receipt of influenza vaccine. |
| IIV=Inactivated Influenza Vaccine; IIV3=Inactivated Influenza Vaccine, Trivalent; IIV4=Inactivated Influenza Vaccine, Quadrivalent; RIV=Recombinant Influenza Vaccine LAIV=Live-Attenuated Influenza Vaccine; IM=intramuscular; ID=intradermal; IN=intranasal.
* Immunization providers should check Food and Drug Administration–approved prescribing information for 2013–14 influenza vaccines for the most complete and updated information, including (but not limited to) indications, contraindications, and precautions. Immunization providers should check Food and Drug Administration–approved prescribing information for 2013–14 influenza vaccines for the most updated, manufacturer-specific information, including (but not limited to) indications, contraindications, and precautions. Package inserts for US-licensed vaccines are available at http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm093833.htm. |
||
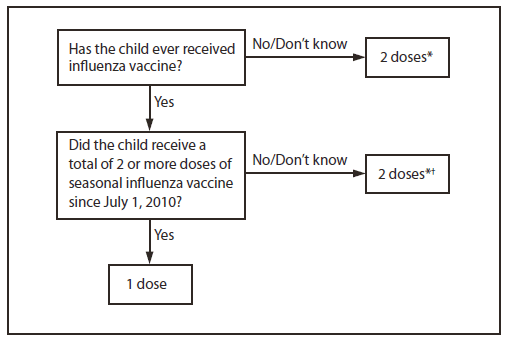
* Doses should be administered at least 4 weeks apart.
† For simplicity, this algorithm takes into consideration only doses of seasonal influenza vaccine received since July 1, 2010. As an alternative approach in settings where vaccination history from before July 1, 2010, is available, if a child aged 6 months through 8 years is known to have received at least 2 seasonal influenza vaccines during any previous season, and at least 1 dose of a 2009(H1N1)-containing vaccine (i.e., 2010–11, 2011–12, or 2012-13 seasonal vaccine or the monovalent 2009[H1N1] vaccine), then the child needs only 1 dose for 2013–14. Using this approach, children aged 6 months through 8 years need only 1 dose of vaccine in 2013–14 if they have received any of the following: 1) 2 or more doses of seasonal influenza vaccine since July 1, 2010; 2) 2 or more doses of seasonal influenza vaccine before July 1, 2010, and 1 or more doses of monovalent 2009(H1N1) vaccine; or 3) 1 or more doses of seasonal influenza vaccine before July 1, 2010, and 1 or more doses of seasonal influenza vaccine since July 1, 2010. Children in this age group for whom one of these conditions is not met require 2 doses in 2013–2014.
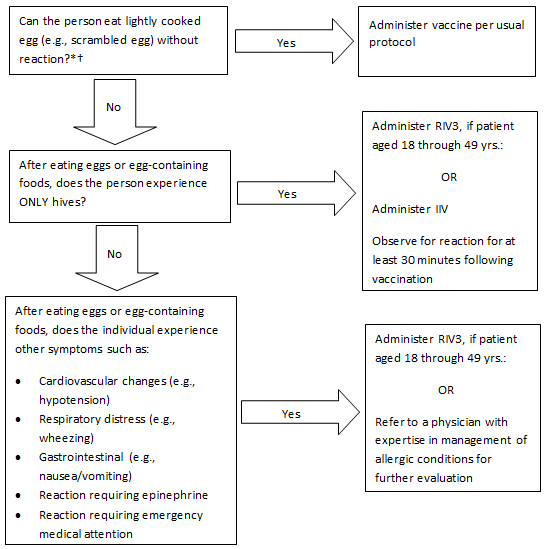
IIV=Inactivated Influenza Vaccine; RIV3=Recombinant Influenza Vaccine, Trivalent
*Individuals with egg allergy may tolerate egg in baked products (e.g. bread, cake). Tolerance to egg-containing foods does not exclude the possibility of egg allergy (2).
† For individuals who have no known history of exposure to egg, but who are suspected of being egg-allergic on the basis of previously performed allergy testing, consultation with a physician with expertise in the management of allergic conditions should be obtained prior to vaccination. Alternatively, RIV3 may be administered if the recipient is aged 18 through 49 years.
References
- Kelso JM, Greenhawt MJ, Li JT, Nicklas RA, Bernstein DI, Blessing-Moore J, et al. Adverse reactions to vaccines practice parameter 2012 update. J Clin All Immunol. 2012 Jul;130(1):25-43.
- Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ. 2009;339:b3680.
- FluMist Quadrivalent [Package Insert]. Gaithersburg, MD: MedImmune; 2013.
- Kroger AT, Sumaya CV, Pickering LK, Atkinson WL. General recommendations on immunization — recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. [Practice Guideline]. 2011 Jan 28;60(2):1-64.
- Centers for Disease Control and Prevention. Update: Recommendations of the Advisory Committee on Immunization Practices (ACIP) Regarding Use of CSL Seasonal Influenza Vaccine (Afluria) in the United States During 2010–11. MMWR 2010; 59(31);989-992.
