CDC Study Describes New Lab Method to Test Flu A(H3N2) viruses, Has Potential to Improve Flu Vaccine Strain Selection
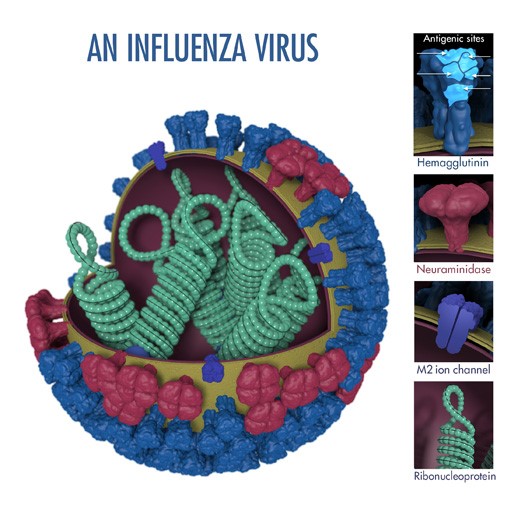
An influenza virus’ surface proteins: hemagglutinin (HA) and neuraminidase (NA) are “antigens,” which are molecular structures that trigger an immune response in an infected host. The term “antigenic properties” is used to describe the characteristics of the immune response triggered by the antigens of a particular virus. “Antigenic characterization” refers to the analysis of different viruses’ antigenic properties to help determine their similarity. Flu experts attempt to choose vaccine viruses for inclusion in seasonal flu vaccines that are antigenically similar or closely “matched” to the viruses most likely to spread and cause illness during the flu season.
March 13, 2019 – A recent study published in Scientific Reports describes CDC’s development and use of a new laboratory method called “HINT” to antigenically characterize 422 influenza A(H3N2) viruses (hereafter called “H3N2”) that circulated mostly in the United States from 2011-2018. Antigenic characterization is a laboratory testing process that flu scientists use to measure how flu viruses are evolving to evade human immunity. This testing is performed year-round as part of global flu surveillance efforts. This data informs recommendations on which vaccine viruses to include in seasonal flu vaccines. HINT, which is short for “high-content imaging-based neutralization test,” has made it possible for scientists to identify specific changes in circulating H3N2 viruses that help the viruses evade immunity associated with vaccination or infection. Antigenic characterization of H3N2 viruses has become increasingly challenging in recent years. The new HINT methodology represents a major step forward in overcoming those challenges, with the potential to speed up and improve selection of candidate vaccine viruses for the H3N2 component of seasonal flu vaccines.
The HI test is the traditional method used by flu scientists to conduct antigenic characterization. It allows circulating flu viruses to be compared with one another and those chosen for use in vaccines. However, in recent years, HI testing has failed to work on current H3N2 viruses. This is because HI testing relies on viruses binding to red blood cells, and most contemporary H3N2 viruses do not bind well to them. Also, the HI test requires that a sufficient amount of flu virus be “raised” for testing. This traditionally has been accomplished by growing the virus in fertilized chicken eggs or cell cultures. However, many current H3N2 viruses do not grow well in chicken eggs. Also, growing H3N2 viruses in the laboratory can result in mutations or changes to the virus that can negatively impact vaccine match.
CDC’s flu scientists developed HINT to work around these problems, and therefore, to improve antigenic characterization of H3N2 viruses. Compared to other currently used assays, HINT offers improved throughput and allows for the possibility of directly analyzing respiratory samples from infected patients, eliminating the need to grow the virus in cell culture. As a result, HINT speeds up antigenic characterization and potentially avoids introducing new mutations or changes to the viruses being characterized. This, together with other programmatic and scientific advances in surveillance, have the collective potential to improve the time required for flu vaccine selection.
The process of selecting flu vaccine viruses and then manufacturing vaccines is a year-round endeavor. For the Northern Hemisphere, flu experts around the world meet in February of each year to compare virus surveillance data and make vaccine component recommendations for the upcoming flu season in the fall. The improvements that HINT brings will allow more timely information to be included in that decision-making process.
HINT provides other benefits as well. This study’s researchers used HINT in combination with new genome sequencing technologies and practices to identify particular evolutionary changes in the HA surface antigen of circulating H3N2 viruses that allow these viruses to evade the human immune response at the molecular level. This study’s authors identified a number of significant mutations in different H3N2 viruses that allowed these viruses to evade the immune protection provided by seasonal flu vaccines. Significant differences between circulating flu viruses and the flu vaccine, which lay audiences sometimes refer to as a “mismatch,” can negatively impact the benefits provided by flu vaccination and can necessitate a change in the vaccine formulation.
Overall, the development and application of the HINT method is an important scientific achievement that supports global efforts to improve the timeliness and accuracy of vaccine virus selection. These advancements demonstrate CDC’s dedication to improving available flu vaccines and to further reduce the burden of flu in the United States and around the world.
