Fatal Gastrointestinal Mucormycosis in an Infant Following Use of Contaminated ABC Dophilus Powder From Solgar Inc.
Highlights
- CDC currently recommends that Solgar ABC Dophilus Powder, a dietary supplement, should not be used.
- CDC, FDA, and state health departments are investigating a fatal case of gastrointestinal mucormycosis caused by Rhizopus oryzae in a premature infant in October 2014. Infection followed the use of ABC Dophilus Powder, a dietary supplement purchased from Solgar, Inc., Leonia, NJ.
- Testing of the same lot of unopened Solgar ABC Dophilus revealed contamination with Rhizopus oryzae. The source of contamination is still under investigation.
- Solgar has voluntarily recalled lots 074024-01R1, 074024-01, 074024-02 (expiration date 7/31/15).
- The investigation is ongoing, and new information will be provided when available.
Description of investigation
CDC, the Food and Drug Administration (FDA), and the Connecticut Departments of Public Health and Consumer Protection are investigating a fatal case of gastrointestinal (GI) mucormycosis in a premature infant born at 29 weeks’ gestation. The infant received Solgar ABC Dophilus lot 074024-01R1 and subsequently developed signs and symptoms consistent with necrotizing enterocolitis (NEC). Surgical exploration of the infant’s GI tract revealed a necrotic bowel, which was resected; histopathology results from the resected bowel revealed a heavy fungal burden with an organism consistent with a mucormycete. Immunohistochemical staining of the tissue block performed locally and at CDC was positive when tested with a monoclonal antibody known to react with several mucormycete fungal agents. Sequencing of fungal DNA from the tissue identified the mucormycete as Rhizopus oryzae.
Solgar ABC Dophilus is a dietary supplement containing viable microbial ingredients. This product is intended to contain three bacteria, Bifidobacterium lactis, Streptococcus thermophilus, and Lactobacillus rhamnosus. This product and other dietary supplements thought to have ‘probiotic’ effects are used in preterm infants on the basis of a recent Cochrane review supporting their use for prophylaxis against NEC.
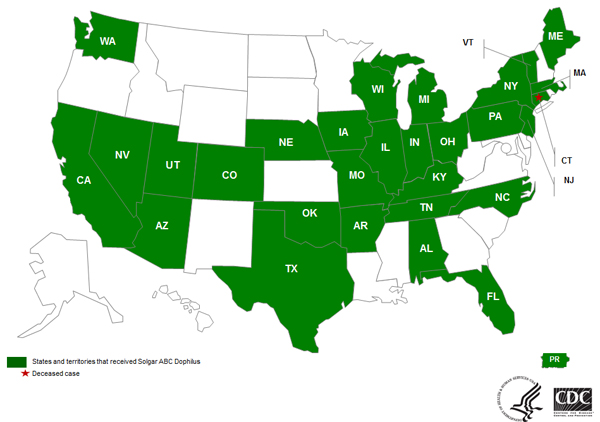
Solgar voluntarily recalled lots 074024-01R1, 074024-01, and 074024-02 (expiration date 7/31/15) on November 14, 2014. This product was distributed domestically to 29 states and Puerto Rico (see map below) and was distributed internationally to the United Kingdom and Israel.
The investigation into this fatal case of GI mucormycosis and its association with the contaminated Solgar ABC Dophilus is ongoing. CDC currently recommends that Solgar ABC Dophilus should not be used, especially in infants who may be more susceptible to infection, until further information regarding the safety of this product is available.
Domestic distribution of recalled Solgar ABC Dophilus
Solgar, Inc.’s ABC Dophilus Powder Distribution Across the United States (29) and Puerto Rico
Laboratory investigation
Local product testing of unopened Solgar ABC Dophilus Powder from lot 074024-01R1 revealed mold growth. Mold from the local product testing was sent to CDC, where it was confirmed to be Rhizopus oryzae, a cause of mucormycosis in humans. CDC laboratory testing of gastrointestinal tissue from the infant also revealed invasive mucormycosis through histopathology and immunochemistry as well as evidence of DNA from Rhizopus oryzae.
Advice to consumers
-
DO NOT use Solgar ABC Dophilus while this investigation is ongoing.
- If consumers find that they have used or unused Solgar ABC Dophilus, they should be aware that lots 074024-01R1, 074024-01, and 074024-02 (expiration date 7/31/15) have been voluntarily recalled by the company with instructions for consumers to return their product to the place of purchase for a full refund.
- Contact your healthcare provider if you are experiencing signs or symptoms consistent with a gastrointestinal mucormycosis infection such as abdominal pain, nausea, or vomiting AND have ingested Solgar ABC Dophilus within the past 30 days.
Advice to healthcare providers
Healthcare facilities and providers should stop using Solgar ABC Dophilus, especially among infants who may be more susceptible to infection with mucormycosis, while this investigation is ongoing. Healthcare providers are asked to report the following to their state or local health departments if they occurred since November 1, 2013:
- Confirmed or suspected cases of infants with gastrointestinal mucormycosis (diagnosed via culture or histopathology) since November 1, 2013, OR
- Unexplained infant deaths within 30 days of receipt of Solgar ABC Dophilus
Clinical signs and symptoms of mucormycosis
Mucormycosis is a rare infection caused by a group of molds called mucormycetes. These fungi are common in the environment. Rhizopus is one of the most common types of mold that causes mucormycosis.
Mucormycosis can affect a variety of organ systems, with rhinocerebral (sinuses and brain) and pulmonary (lung) infections being the most common forms. In general, the symptoms of mucormycosis depend on the body site where the infection occurs. The clinical hallmark of mucormycosis is the rapid onset of tissue necrosis (tissue death) with or without fever. Necrosis is the result of invasion of blood vessels and subsequent thrombosis (blood clotting).
Gastrointestinal mucormycosis is thought to occur after a person ingests the mold, and it usually affects persons who do not have the normal ability to fight off infection, such as severely malnourished people or transplant recipients. The stomach, colon, and ileum are the most commonly involved sites. The symptoms may be very non-specific and include abdominal pain and swelling, nausea, and vomiting.
More Information
- Notes from the Field: Fatal Gastrointestinal Mucormycosis in a Premature Infant Associated with a Contaminated Dietary Supplement – Connecticut, 2014. MMWR. 2015 Feb 20;64(6):155-6.
- FDA Investigates Presence of Mucormycosis-causing Mold in Infant and Children’s Probiotic Supplement
- FDA – Solgar, Inc. Issues Voluntary Class I Recall of ABC Dophilus Powder
- CDC – Mucormycosis
- The Cochrane Collaboration: Probiotics for prevention of necrotizing enterocolitis in preterm infants