Global Health Security Agenda: GHSA Antimicrobial Resistance Action Package (GHSA Action Package Prevent-1)
As Measured by:
(1) Number of comprehensive plans to combat antimicrobial resistance agreed and implemented at a national level, and yearly reporting against progress towards implementation at the international level
(2) Number of countries actively participating in a twinning framework, with countries agreeing to assist other countries in developing and implementing comprehensive activities to combat antimicrobial resistance, including use of support provided by international bodies to improve the monitoring of antimicrobial usage and resistance in humans and animals.
Desired National Impact: Decisive and comprehensive action to enhance infection prevention and control activities to prevent the emergence and spread of AMR, especially among drug-resistant bacteria. Nations will strengthen surveillance and laboratory capacity, ensure uninterrupted access to essential antibiotics of assured quality, regulate and promote the rational use of antibiotics in human medicine and in animal husbandry and other fields as appropriate, and support existing initiatives to foster innovations in science and technology for the development of new antimicrobial agents.
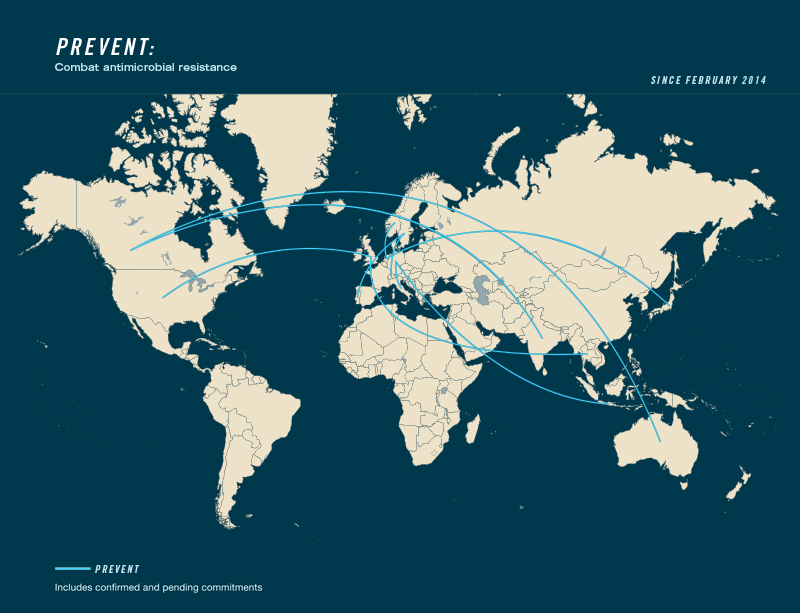
Country Commitments to Action Package:
- Leading countries: Canada, Germany, Netherlands, Sweden, United Kingdom
- Contributing countries: Australia, India, Indonesia, Italy, Japan, Norway, Portugal, Switzerland, Thailand, United States
- Contributing international organizations: FAO, OIE, WHO
Five-Year Action Items:
Consistent with the WHO process to coordinate development of an AMR Global Action Plan:
- Develop a national action plan, based on a one health approach, to combat antimicrobial resistance which includes:
– best practice in hygiene and infection prevention measures,
– the presence of reliable surveillance systems for antimicrobial resistances and antibiotic consumption, considering the different sectors: hospitals, outpatient facilities and the community as well as in veterinary medicine and in animal husbandry,
– uninterrupted access to essential and affordable antibiotics of assured quality,
– measures for controlled and restrained use of antimicrobials,
– actions to raise awareness of AMR issues in order to improve the effective use of antibiotics by public and professionals,
– measures that minimize the occurrence of residues of antibiotics and the spread of resistant bacteria in(to) the environment. - Develop and implement guidelines and standards for infection prevention.
- Develop and use new guidelines or encourage use of existing guidelines, training and other resources to promote the prudent and restrained use of antimicrobials, in both human, animals and other areas when appropriate.
- Ensure access to at least one reference laboratory for each country capable of identifying at least three of the seven WHO priority AMR pathogens using standardized, reliable detection assays, and reporting these results. Alternatively one reference laboratory for each of the three priority pathogens should be in place.
- Support ongoing work with international organizations to develop and implement a harmonized approach for monitoring and surveillance of antimicrobial drug use and antimicrobial resistance in humans and animals, including interpretive criteria for susceptibility reporting across WHO, FAO, and OIE regional surveillance programs. Using this information, these organizations can strengthen outreach activities to influence health professionals (veterinary and human) on the need for early recognition of AMR and prudent use of antimicrobials to limit the spread of AMR.
- Collaboration in international initiatives to encourage and accelerate the discovery and development of new generations of antibiotics, including new models for controlled distribution and use of these new antibiotics.
- Collaboration in international initiatives to encourage and accelerate the discovery and development of inexpensive and rapidly deployable, point-of-care diagnostics.
- Identification of relevant stakeholders spanning human, animal, agricultural, food and environmental aspects
- Analysis of the present status on the national level and identification of areas that need to be strengthened (e.g. surveillance systems, capacity and capability of existing laboratories for the diagnosis of AMR)
- Identification of guidelines (e.g. from WHO) that can be adapted to the situation in the country
- Use WHO’s IHR Monitoring Framework, OIE’s PVS Pathway, and other appropriate instruments to identify countries’ priorities for strengthening core competencies.
- 1.Introduce arrangements which are consistent with the WHO process to coordinate development of an AMR Global Action Plan