A Giant Step toward Polio Eradication
Imagine getting 155 countries and territories to agree on anything.
Then imagine getting 155 countries and territories sufficiently organized, coordinated, and invested to simultaneously switch the oral polio vaccine used at every one of their health facilities providing vaccines from one type to another. While it sounds almost impossible, public health agencies and health professionals around the globe have made this astounding feat a reality, and in the process they have moved the world one step closer to eradicating polio.
Accomplishing the switch highlighted an unglamorous, but crucially important, side of the everyday existence of CDC and its partners in public health – the ability to develop and master complex logistics. The ability to work with a collection of different global organizations and cadres of health professionals with diverse opinions and philosophies, all in the pursuit of keeping people safe from disease, is often overshadowed by CDC’s science. But without it, campaigns to protect public health and save lives rarely succeed.
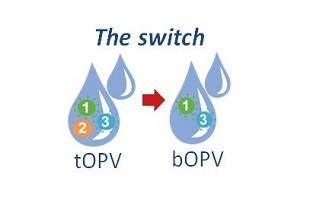
The origins of the switch in oral polio vaccines began with discoveries made by scientists at the CDC polio laboratory in the late 1990s and early 2000s that showed that the weakened type 1, 2, and 3 polioviruses in trivalent oral polio vaccine (tOPV) could, in very rare cases, cause outbreaks of paralytic polio.
According to CGH Global Immunization Division’s Lee Hampton, MD, oral polio vaccine contains domesticated polioviruses that protect against infection with wild polioviruses (WPVs). Using oral polio vaccine is similar to using a dog like a German shepherd to protect against wolves. In rare cases the domesticated vaccine virus becomes feral and acts like the wild virus, like a dog that joins a wolf pack. Although oral polio vaccine has been in use since the 1960s, the risks posed by these feral vaccine derived polioviruses (VDPVs) were not recognized until work by CDC scientists Olen Kew, Cara Burns, Jing Shaw, Jaume Jorba, Steve Oberste, Mark Pallansch, and other CDC scientists allowed VDPVs to be genetically distinguished from WPVs.
Working with colleagues around the world, CDC polio virologists and epidemiologists like Steve Wassilak have shown that since 2000 more than 800 cases of polio have been caused by VDPVs; approximately 86% were from type 2 VDPVs. Trivalent oral polio (tOPV) vaccine has been key to polio eradication efforts, but, as the world nears eradicating polio, the vaccine’s risks have begun to edge out its value. Global polio experts concluded about four years ago that the world needed to switch from tOPV to a bivalent oral polio vaccine (bOPV), which contains only types 1 and 3 polioviruses, to stop cases of polio caused by type 2 VDPVS from occurring and to get over the finish line to eradicate polio.
It’s a simple idea that’s complex for one reason – every country must achieve high immunity to type 2 polio infections and then switch at the same time. If they don’t, type 2 polio could burst to life much like a wildfire in a forest that was bypassed by firefighters. Continued use of tOPV in some areas but not others after the switch would be like playing with matches in a forest. Dr. Hampton appreciates the firefighting analogy and takes it a step further.
Switching the polio vaccine successfully, he says, is like putting out all wildfires everywhere at the same time but then stopping use of the primary means of fighting the fires (tOPV) because that tool can occasionally cause fires.
Once the science showed the value of switching, the planning began.
The four-year planning period by numerous partners and organizations gave the Global Polio Eradication Initiative (GPEI), of which WHO, UNICEF, Rotary International, the Bill and Melinda Gates Foundation, and CDC are the core partners, time to convince countries that this switch was necessary. GPEI built on its long-standing work and resultant credibility in many countries, with WHO and UNICEF regional and country offices providing tremendous support. All countries agreed to make the switch in April 2016. By May 12, 2016 all 155 countries that were using or stockpiling tOPV in 2015 had removed tOPV from use in their immunization programs.
As part of the preparations for the switch, GPEI worked with countries to make sure that all countries had the highest possible population immunity to type 2 polio infections prior to the switch. In addition to efforts to strengthen delivery of vaccines through national immunization programs and to provide tOPV in mass vaccination campaigns, GPEI worked with Gavi, The Vaccine Alliance, to help all countries begin using inactivated poliovirus vaccine (IPV) before the switch. One of the challenges has been a shortage of IPV for this effort caused by delays in vaccine production. The IPV provides some immunity against poliovirus type 2, especially paralytic polio caused by poliovirus type 2, and boosts immunity against polio types 1 and 3 (keeping the forest as damp as possible after the switch with a weaker but safer fire suppressant in Hampton’s forest analogy). However, the limited supply of IPV means that some countries can’t start using IPV until 2017.
A key step in preparing for the switch was getting manufacturers to agree to stop making tOPV. If one company had decided to keep selling it, that would have created a huge problem, a stockpile of matches ready to start a fire in a dry forest. Fortunately, GPEI, especially WHO and UNICEF, convinced manufacturers to stop making it. This became particularly important when, in the last few days before the switch, a country that had not yet received IPV pushed to keep using tOPV. Because it could not find additional supplies of tOPV, it ran out of it and switched to bOPV soon after the other countries instead of putting the world at risk of additional type 2 VDPV outbreaks.
To make sure that all tOPV had been removed from use, independent monitors from universities, NGOs, and other groups checked health facilities, and especially national vaccine storage facilities, to see that they had gotten rid of their tOPV. To date, all 155 countries and territories have started independent monitoring of their completion of the switch, and well over 160,000 vaccine stores and health facilities have been visited by monitors. As of August 25, 2016, 153 countries and territories have sent reports to WHO regional offices validating that the switch has been accomplished. Working with its GPEI partners to set up the monitoring to validate compliance with the switch was one of CDC’s main contributions to the implementation of the switch. Without these data, there would be no way of tracking progress and identifying and quickly addressing problems.
Dr. Hampton was among the CDC staff who went to various countries as an observer to help organize the monitoring of the switch. The following staff were directly involved in monitoring the switch in their respective countries: Roodly Archer, Madagascar; Gabriel (Gabe) Anaya, Cambodia; Emily (Ly) Cloessner, Madagascar; Samantha (Sam) Dolan, Uganda; Daniel Ehlman, Ecuador; Lee Hampton, Sierra Leone; Jennifer (Jennie) B. Harris, Guinea; Mary Huynh, Sierra Leone; Carla Lee, Equatorial Guinea; Mahnaz Motevalli Oliner, Sierra Leone; Jenny Walldorf, Sierra Leone; Cheryl Williams, Haiti; and John Yembu, Haiti.
Many other CDC secondees and staff from Atlanta and country offices were crucial to this effort, building on the infrastructure already put in place by GPEI and other public health organizations. Among them were Manish Patel, seconded to the Task Force for Global Health; Lance Rodewald, seconded to WHO-China; Will Schulter, seconded to the Western Pacific Regional office of WHO; Terri Hyde, CDC-Atlanta; Steve Wassilak, CDC-Atlanta; Allen Craig, CDC-Atlanta; Fabien Diomande, CDC-Atlanta; Edgar Garcia, CDC-Atlanta; Olen Kew, CDC-Atlanta, and Cara Burns, CDC-Atlanta.
As complicated as this switch in oral polio vaccines was, it has some precedent in CDC’s history. There are some similarities between the polio switch and the rollout of the H1N1 flu vaccine during the 2009 flu pandemic. Just as in 2009, the tOPV to bOPV switch built on an existing vaccine distribution system to a great extent, and cooperation from many jurisdictions was needed to distribute the vaccine. The big differences between the switch and the H1N1 pandemic flu vaccine in 2009 are that the switch involved distributing bOPV as tOPV was simultaneously withdrawn, that bOPV was not a completely new vaccine, and that there was much more time to prepare for the switch than for the distribution of the H1N1 vaccine.
Several countries have identified type 2 VDPVs among their populations since tOPV use stopped – think of it as an ember left in the forest that could flare up. While these VDPV2s should be controllable with oral polio vaccine containing type 2 poliovirus (the forest has not completely dried out yet), additional VDPV outbreaks could occur in the future if a country decided to use tOPV in the future – lighting matches in a dry forest. Ultimately, the success of the switch will be measured by how many type 2 VDPV virus cases there are in the future; the goal, of course, is zero cases. One or two years without additional cases of polio caused by VDPV2s would likely signal success. Having many more cases of polio caused by VDPV2s would be a real concern.
Disease surveillance is always crucial because of the possibility of future polio cases. The polio surveillance system is built around the global polio laboratory network, which can sequence polioviruses and find out where the virus originated. The surveillance system can determine if type 2 poliovirus originated from vaccine, and if it is from the embers – VDPVs that existed at the time of the switch, or from vaccine used after the switch. The massive polio vaccine campaigns before the switch hopefully doused most of the embers. A response plan is in place along with a stockpile of monovalent vaccine for type 2 polio virus, for any type 2 cases that occur after the switch.

John Yembu (CDC/OPHPR/DEO) with tOPV at Commune de Cornillon, Haiti
The surveillance officers who track down patients with possible polio and collect stool samples from them for poliovirus testing are another key part of the polio surveillance system. In dozens of countries they also collect environmental (sewage) samples that can show if poliovirus is present in a population even if no cases of polio have been detected, which is especially useful in the context of conflict or a natural disaster. In even more countries, they also help organize mass vaccination campaigns against polio and to track down children who need to be vaccinated.
Reaching goals in vaccination campaigns means assistance comes in various forms. In some countries, such as Pakistan, assistance with the vaccine campaign can include police protection from the Taliban as well as hoping the army can stop the Taliban from stopping the vaccine campaign in areas they control. In other countries, negotiations for a temporary peace (Day of Tranquility) to let the vaccinations take place sometimes occur.
Despite all of the challenges, including the recent detection of type 2 wild polioviruses in Nigeria for the first time in two years, eradication of polio altogether is still the goal. One part of that is phasing out bOPV in a few years. A key question for the polio endgame is “How long do we keep vaccinating with IPV after there are no more cases?” CDC is heavily involved in finding the answer to that question, including efforts to develop a super-domesticated oral polio vaccine that’s far less likely to cause VDPV. Think of it as super fire suppressant to permanently douse polio without the risk of causing polio itself.
CGH Director Rebecca Martin, PhD, long involved in polio eradication, spoke of the personal investment of prevention: “When you know you’ve made a difference for a child, a family, a community, a country, the momentum builds to make sure everyone is vaccinated against polio.” Dr. Martin also noted that the decades of work on eradicating polio have established global working relationships, clinics, labs, and data collection that are in place for the next disease outbreak. This infrastructure has already proven useful for efforts to combat other infectious diseases. For example, the polio infrastructure in Nigeria played a key role in stopping the spread of Ebola viral disease there in 2014.
That the goal of eradicating polio can be reached in our lifetime is an unbelievably bright moment for all of those who gave money or time to make the development and use of polio vaccines possible, stood in lines to get sugar cubes with the vaccine in them, watched schoolmates stricken with polio deal with its lifelong effects, and/or watched it devastate entire generations of children worldwide. For the legions of scientists at CDC and at public health agencies around the world, achieving this will rank as one of the greatest achievements in global public health.