Interim Guidance for the Detection of Novel Influenza A Virus Using Rapid Influenza Diagnostic Tests
August 10, 2009
This guidance was revised to clarify that the current rRT-PCR developed by CDC to detect novel influenza A ( H1N1) is authorized by the FDA. The FDA authorization, also termed Emergency Use Authorization or EUA, is not equivalent to FDA cleared, which was incorrectly stated in the previous version of the guidance.
Background
This interim guidance provides an overview of the sensitivities of rapid influenza diagnostic tests (RIDT) in detecting novel influenza A (H1N1) virus in order to help guide the reporting and interpretation of test results. This document does not discuss either direct (DFA) or indirect immunofluorescence assays (IFA). This guidance is primarily intended for clinical laboratories and clinical practices conducting influenza testing on respiratory specimens from patients with suspected novel influenza A (H1N1) virus infection. Information on laboratory biosafety is available as well as interim guidance on clinical testing recommendations. Guidance will be updated as needed based on new data.
Influenza Diagnostic Tests
A number of different laboratory diagnostic tests can be used for detecting the presence of influenza viruses in respiratory specimens, including direct antigen detection tests, virus isolation in cell culture, or detection of influenza-specific RNA by real-time reverse transcriptase-polymerase chain reaction (rRT-PCR). These tests differ in their sensitivity and specificity in detecting influenza viruses as well as in their commercial availability, the amount of time needed from specimen collection until results are available, and the tests’ ability to distinguish between different influenza virus types (A versus B) and influenza A subtypes (e.g. novel H1N1 versus seasonal H1N1 versus seasonal H3N2 viruses). Serologic tests on paired acute (within 1 week of illness onset) and convalescent (collected 2-3 weeks later) sera can help to establish a retrospective diagnosis of influenza virus infection for epidemiological and research studies. However, such serial serological testing is not routinely available through clinical laboratories. At this time, there are only two FDA authorized assays for confirmation of novel influenza A(H1N1) virus infection, including the CDC rRT-PCR Swine Flu Panel assay; however, other rRT-PCR assays such as laboratory developed tests, not approved by FDA, may be able to detect novel influenza A (H1N1) viruses. Public health laboratories in the U.S. are able to perform the CDC rRT-PCR Swine Flu Panel assay. Confirmation of novel influenza A(H1N1) infection may be necessary for surveillance purposes and for special situations, e.g. severely ill patients, patients with immunocompromising conditions, and pregnant and breast feeding women. State and local jurisdictions dictate the specific influenza assay results required for influenza surveillance.
Rapid Influenza Diagnostic Tests
Rapid influenza diagnostic tests (RIDTs) are antigen detection tests that detect influenza viral nucleoprotein antigen. The commercially available RIDTs discussed in this document can provide results within 30 minutes or less. Thus, results are available in a clinically relevant time period to inform clinical decisions. These assays may be referred to as “point-of care” tests since CLIA-waived RIDTs (not all RIDTs are CLIA waived) may be used in facilities with a certificate of waiver or in locations outside a central laboratory. Commercially available RIDTs can either: i) detect and distinguish between influenza A and B viruses; ii) detect both influenza A and B but not distinguish between influenza A and B viruses; or, iii) detect only influenza A viruses. None of the currently FDA approved RIDTs can distinguish between influenza A virus subtypes (e.g. seasonal influenza A (H3N2) versus seasonal influenza A (H1N1) viruses), and RIDTs cannot provide any information about antiviral drug susceptibility. For detection of seasonal influenza A virus infection in respiratory specimens, RIDTs have low to moderate sensitivity compared to viral culture or RT-PCR. The sensitivities of RIDTs to detect influenza B viruses are lower than for detection of influenza A viruses. The sensitivities of RIDTs appear to be higher for specimens collected from children than specimens collected from adults.
Few comparisons of RIDTs with RT-PCR for the detection of novel influenza A (H1N1) virus or seasonal influenza viruses have been published. Three recent analytical studies indicate that commercially available RIDTs are reactive with the nucleoprotein of novel influenza A (H1N1) virus.1 However, only limited data have been published on the performance of RIDTs compared with RT-PCR for detecting the presence of novel influenza A (H1N1) virus in clinical specimens.2 Compared to RT-PCR, the sensitivity of RIDTs for detecting novel influenza A (H1N1) virus infections ranged from 10-70%1. Therefore, a negative RIDT result does not rule out novel influenza A (H1N1) virus infection. While limited by small numbers, currently published side-by-side comparisons of RIDTs to detect novel influenza A (H1N1) and seasonal influenza A viruses suggest the sensitivity of RIDTs to detect novel influenza A (H1N1) virus is equal to or lower than the sensitivity to detect seasonal influenza viruses2. Factors that might contribute to a lower sensitivity for influenza laboratory tests to detect novel influenza A (H1N1) virus infection include the type of respiratory specimen (i.e., nasal vs. nasopharyngeal swab), quality of the specimen, time from illness onset to specimen collection, the age of the patient, time from specimen collection to testing, and the storage and processing of the specimen prior to testing.
The Role of RIDT for Detecting Novel H1N1: Clinical Considerations
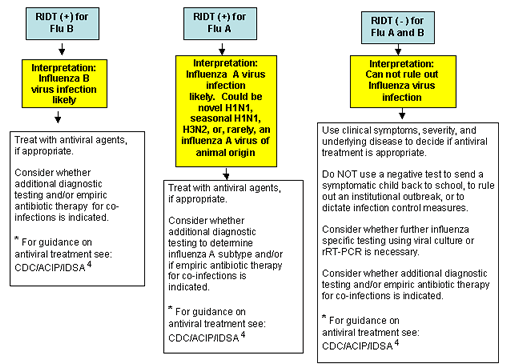
A RIDT may provide useful information that might impact on patient care (Figure). However, understanding the limitations of RIDTs is very important to appropriately interpret results for clinical management.3 When influenza viruses are circulating in a community, a positive test result indicates that influenza virus infection is likely present in the specimen. Knowledge of the presence of influenza A or B virus infection can help to inform influenza treatment decisions. However, a negative rapid test result does not rule out influenza virus infection. Since false negative results can occur, if clinical suspicion of influenza is high in a patient who tests negative by RIDT (or if RIDT is not offered), empiric antiviral therapy should be administered, if appropriate, and infection control measures implemented. Guidance on the use of influenza antiviral agents is available. In settings where policies indicate exclusion of patients who may have influenza (e.g., schools, camps, day care centers), a negative RIDT, performed on a patient with clinically compatible illness, should not be used as justification for early return to that setting. Finally, a negative RIDT result can not exclude influenza as a cause of an outbreak in a facility with ill residents or patients with clinically compatible illness.
The specificity of RIDTs is generally high. However, especially during periods of low influenza activity (e.g. the very beginning of the season), false positive results can occur. During low influenza activity periods, confirmation of positive RIDT results by another testing method such as viral culture or rRT-PCR should be considered.
If laboratories are performing RIDTs, it would be prudent to add a statement about the test limitations in the report of results so that the physician can decide how best to use the test for patient management.
Example of a Statement to Accompany Rapid Influenza Diagnostic Test Results
RIDT result: Positive for Influenza Type A
Note: This test can not distinguish influenza A virus subtypes. For example, this test cannot distinguish influenza infections caused by novel influenza A viruses versus seasonal influenza A viruses.RIDT result: Negative for Influenza A and B
Note: The sensitivity of this assay has been shown to range between [10-70%*] for the detection of novel influenza A (H1N1) virus and between [20-100%*] for seasonal influenza viruses. A negative result does not exclude influenza virus infection. If influenza is circulating in your community, a diagnosis of influenza should be considered based on a patient’s clinical presentation and empiric antiviral treatment should be considered, if indicated. If more conclusive testing is desired, follow-up confirmatory testing with either [viral culture or RT-PCR*] is warranted.* Fill in with individual clinical laboratory data and information
Figure. Algorithm to assist in the interpretation of RIDT results during periods when influenza viruses are circulating in the community
1 Hurt AC et al. Performance of influenza rapid point-of-care tests in the detection of swine lineage A(H1N1) influenza viruses. Influenza and Other Respiratory Viruses 2009;3(4):171-76;
Chan KH et al. Analytical sensitivity of rapid influenza antigen detection tests for swine-origin influenza virus (H1N1). J Clin Virol. 2009 Jul;45(3):205-7; CDC. MMWR 2009;in-press
2 Faix DJ, Sherman SS, Waterman SH. Rapid-Test Sensitivity for Novel Swine-Origin Influenza A (H1N1) Virus in Humans. N Engl J Med. 2009 Jun 29 [Epub ahead of print];
Ginocchio CC et al. Evaluation of multiple test methods for the detection of the novel 2009 influenza A (H1N1) during the New York City outbreak. J Clin Virol. 2009 Jul;45(3):191-5. CDC. MMWR 2009; in-press
3 Uyeki TM. Influenza diagnosis and treatment in children: a review of studies on clinically useful tests and antiviral treatment for influenza. Pediatr Infect Dis J. 2003 Feb;22(2):164-77.
4 * CDC: Updated Interim Recommendations for the Use of Antiviral Medications in the Treatment and Prevention of Influenza for the 2009-2010 Season
Fiore AE et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recommendations and Reports 2009; in-press
Harper SA et al. Seasonal Influenza in Adults and Children - Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management: Clinical Practice Guidelines of the Infectious Diseases Society of America. Clinical Infectious Diseases 2009; 48:1003–32.
Get email updates
To receive weekly email updates about this site, enter your email address:
Contact Us:
- Centers for Disease Control and Prevention
1600 Clifton Rd
Atlanta, GA 30333 - 800-CDC-INFO
(800-232-4636)
TTY: (888) 232-6348 - Contact CDC-INFO