Updated Interim Recommendations for the Use of Antiviral Medications in the Treatment and Prevention of Influenza for the 2009-2010 Season
December 07, 2009 5:00 PM ET
On this Page
- Objective
- Introduction
- Treatment of confirmed or suspected influenza
- Antiviral Chemoprophylaxis of exposed individuals
- Antiviral drug use for control of influenza outbreaks in institutions
- Specific regimens for treatment and chemoprophylaxis of influenza
- Antiviral prescription and dispensing considerations
- Adverse events and contraindications
- Further information
These recommendations contain the following updates:
- Information regarding use of intravenous peramivir under an emergency use authorization
- Information on availability of renal dosing for peramivir
- Updated oseltamivir dosing instructions for children younger than 1 year of age based on weight
- Antiviral treatment and chemoprophylaxis considerations for patients vaccinated with 2009 H1N1 and seasonal influenza vaccines
- Guidance on early empiric antiviral treatment for patients with progressive or severe influenza-like illness, regardless of underlying medical conditions
- Guidance on early empiric antiviral treatment patients with underlying medical conditions placing them at risk for complications
- Clarification of treatment considerations for patients with illness longer than 48 hours
These recommendations focus on the use of antiviral medications for the treatment and chemoprophylaxis of influenza. As of December 4, 2009, influenza A (H1N1) virus (2009 H1N1), is the strain responsible for >99% of influenza in the U.S. during the 2009-2010 influenza season. For information about other influenza viruses refer to CDC's Website. These recommendations were last updated on December 4, 2009, and will be updated periodically as new information becomes available.
Objective
To provide updated recommendations on the use of antiviral agents for treatment and prophylaxis of influenza during the 2009-2010 influenza season.
Introduction
Through November, 2009, ~99% of typed influenza viruses have been 2009 H1N1. The vast majority of 2009 H1N1 viruses tested for drug resistance have been susceptible to oseltamivir and zanamivir but resistant to the adamantanes (amantadine, rimantadine). Surveillance data, updated weekly, are available at Weekly Flu View. These recommendations will be revised as needed to adapt to new information on risk factors, antiviral availability and resistance, or the circulation of other influenza viruses.
In general, treatment with an antiviral agent, when indicated, should begin as soon as possible after the onset of typical influenza-like symptoms. Influenza illness can present in a range of symptomatology: from a mild upper respiratory infection to an acute, life-threatening illness.
- Mild or uncomplicated illness is characterized by typical symptoms like fever (although not everyone with influenza will have a fever), cough, sore throat, rhinorrhea, muscle pain, headache, chills, malaise, sometimes diarrhea and vomiting, but no shortness of breath and little change in chronic health conditions.
- Progressive illness is characterized by typical symptoms plus signs or symptoms suggesting more than mild illness: chest pain, poor oxygenation (e.g. tachypnea, hypoxia, labored breathing in children), cardiopulmonary insufficiency (e.g. low blood pressure), CNS impairment (e.g. confusion, altered mental status), severe dehydration, or exacerbations of chronic conditions (e.g. asthma, chronic obstructive pulmonary disease, chronic renal failure, diabetes or other cardiovascular conditions).
- Severe or complicated illness is characterized by signs of lower respiratory tract disease (e.g., hypoxia requiring supplemental oxygen, abnormal chest radiograph, mechanical ventilation), CNS findings (encephalitis, encephalopathy), complications of low blood pressure (shock, organ failure), myocarditis or rhabdomyolisis, or invasive secondary bacterial infection based on laboratory testing or clinical signs (e.g. persistent high fever and other symptoms beyond three days).
Influenza viruses are transmitted from person to person primarily through contact with infected respiratory secretions, especially airborne droplets generated by coughing and sneezing. Viral replication and shedding are key considerations in the timing of treatment, infection control, and chemoprophylaxis. In general, the incubation period for influenza is estimated to range from 1 to 4 days with an average of 2 days. Influenza virus shedding (the time during which a person might be infectious to another person) begins the day before illness onset and can persist for 5 to 7 days, although some persons may shed virus for longer periods, particularly young children and severely immunocompromised persons. The amount of virus shed is greatest in the first 2-3 days of illness and appears to correlate with fever, with higher amounts of virus shed when temperatures are highest. For these recommendations, however, the infectious period for influenza is defined as one day before fever begins until 24 hours after fever ends.
Treatment of Confirmed or Suspected Influenza
Who to treat
Prompt empiric treatment is recommended for persons with suspected or confirmed influenza and:
- Illness requiring hospitalization
- Progressive, severe, or complicated illness, regardless of previous health status, and/or
- Patients at risk for severe disease (see below for groups at high risk)
How to treat
- Antiviral drugs: oseltamivir (oral), zanamivir (inhaled)
- For detailed dosing guidelines, see table 1 below
- Initiate treatment as early as possible after onset of symptoms
- Treat empirically before diagnostic test results are reported
- When definitive diagnosis is indicated, request definitive diagnostic tests (rRT-PCR*, viral culture) rather than rapid tests (RIDT*, DFA*)
* rRT-PCR: real-time reverse transcriptase polymerase chain reaction; RIDT: rapid influenza diagnostic test, DFA: direct immunofluorescence assay
Antiviral Drugs for Treatment of Influenza
The neuraminidase inhibitors are the drugs of choice for treatment of 2009 H1N1 influenza and influenza-like illness in both children and adults in the U.S. at this time.
Oseltamivir - The neuraminidase inhibitor oseltamivir formulated as capsules or oral suspension (Tamiflu®) is FDA-approved for the treatment of uncomplicated acute influenza in patients 1 year and older who have been symptomatic for no more than 2 days. The FDA has issued an Emergency Use Authorization (EUA) authorizing treatment with oseltamivir of patients less than 1 year old with 2009 H1N1 influenza. In addition, the EUA authorizes treatment of patients symptomatic with 2009 H1N1 influenza for more than 2 days and patients sick enough to require hospitalization (see www.cdc.gov/h1n1flu/eua/tamiflu.htm).
Zanamivir - The neuraminidase inhibitor zanamivir formulated for oral inhalation (Relenza®) is FDA-approved for the treatment of influenza in patients 7 years of age and older who, similar to approved uses for oseltamivir, have uncomplicated illness and have been symptomatic for no more than 2 days. As with oseltamivir, the FDA has issued an EUA authorizing treatment with zanamivir of patients with 2009 H1N1 influenza who have been symptomatic for more than 2 days and patients sick enough to require hospitalization (see www.cdc.gov/h1n1flu/eua/relenza.htm ).
Peramivir - A third neuraminidase inhibitor peramivir formulated for intravenous (IV) administration is an investigational product currently being evaluated in clinical trials. As of October, 2009, safety and/or efficacy data from 1,891 patients with acute uncomplicated seasonal influenza A has been submitted to the FDA. Efficacy and safety have not been evaluated in hospitalized patients. Even though the data are insufficient to allow FDA approval, the FDA issued an EUA for treatment with peramivir of hospitalized patients with 2009 H1N1 influenza who have potentially life-threatening suspected or laboratory confirmed infection. Peramivir IV is available through the CDC upon request of a licensed physician. Under the EUA, treatment of adult patients with IV peramivir is approved only if: (1) the patient has not responded to either oral or inhaled antiviral therapy; (2) drug delivery by a route other than IV is not expected to be dependable or is not feasible; or (3) the clinician judges IV therapy is appropriate due to other circumstances. Treatment of pediatric patients is approved if either of the first two criteria apply.
Patients who have severe, complicated, or progressive illness or who are hospitalized
Treatment is recommended for patients with confirmed or suspected 2009 H1N1 influenza who have severe, complicated, or progressive illness or who are hospitalized. The recommended duration of treatment is 5 days. Hospitalized patients with severe infections (such as those with prolonged infection or who require intensive care unit admission) might require longer treatment courses. Even though treatment is most effective when started in the first 48 hours of illness, limited data from observational studies of hospitalized patients suggests treatment of persons with prolonged or severe illness reduces mortality or duration of hospitalization even when treatment is started more than 48 hours after onset of illness. Antiviral doses recommended for treatment of 2009 H1N1 influenza in adults or children 1 year of age or older are the same as those recommended for seasonal influenza (Table 1). Some experts have advocated use of doubled doses of oseltamivir for some severely ill patients, although there are no published data demonstrating that higher doses are more effective. For patients unable to take oral medication or in whom oral medication appears to be ineffective, peramivir for intravenous administration is available from the CDC under an FDA EUA, although studies of efficacy and safety are limited. For further information on treatment of hospitalized patients see, “Antiviral Treatment Options, including Intravenous Peramivir, for Treatment of Influenza in Hospitalized Patients for the 2009-2010 Season” and “Updated Recommendations for Health Care Providers of Children and Adolescents on the Use of Antiviral Medications for the Management of 2009 H1N1 and Seasonal Influenza for the 2009-2010 Season”.
Patients at Increased Risk for Complications
Prompt empiric antiviral drug treatment is recommended for persons with confirmed or suspected influenza who are at increased risk for serious morbidity and mortality. Based on currently available data, approximately 70% of persons hospitalized with 2009 H1N1 are in one or more of the following groups:
- Children (see below) younger than 2 years old
- Adults 65 years of age or older
- Pregnant women and women up to 2 weeks postpartum (regardless of how the pregnancy ended [live birth, pregnancy termination, preterm birth, miscarriage, fetal death])
- Persons with certain medical conditions, described below.
Children: Children younger than 2 years of age are at higher risk for influenza-related complications and have a higher rate of hospitalization compared to older children. Children aged 2 to 4 years are more likely to require hospitalization or urgent medical evaluation for influenza compared with older children and adults, although the risk is much lower than for children younger than 2 years old. From April through September 2009 hospitalization rates for laboratory-confirmed 2009 H1N1 influenza were 4.5-fold higher among children < 2 years of age, 2-fold higher among children 2-4 years of age, and 1.6-fold higher among children 5-17 years of age than among adults (see Weekly Flu View). In April, 2009, the FDA authorized oseltamivir use in children younger than 1 year under an Emergency Use Authorization (EUA) in response to the current public health emergency involving 2009 H1N1 influenza. Use of oseltamivir in children younger than 1 year is subject to the terms and conditions of the EUA. Retrospective safety data on oseltamivir treatment of seasonal influenza in children younger than 1 year old are limited and suggest that severe adverse events are rare. Prospective data continue to be collected on safety and efficacy of oseltamivir in this age group. Dosing for children younger than 1 year is based on the EUA guidance. Details are provided in Table 1, below. (See also: Emergency Use Authorization of Tamiflu (oseltamivir) available at /h1n1flu/eua/). Children and adolescents under 19 years of age who are receiving long-term aspirin therapy are also at increased risk. For more detailed information, see “Updated Recommendations for Health Care Providers of Children and Adolescents on the Use of Antiviral Medications for the Management of 2009 H1N1 and Seasonal Influenza for the 2009-2010 Season”.
Adults aged 65 years and older: Even though persons aged 65 years and older are less likely to become ill with 2009 H1N1 influenza compared to younger persons, when they do acquire influenza, they are at higher risk for severe influenza-related complications.
Pregnant women: Pregnancy increases the risk of complications, hospitalization, and severe disease. One study estimated the risk of hospitalization for 2009 H1N1 to be four times higher for pregnant women than for the general population (Jamieson DJ, et al. Lancet. 2009;374:451-458). While oseltamivir and zanamivir are "Pregnancy Category C" medications, meaning no clinical studies have been conducted to assess the safety of these medications for pregnant women, available data suggest pregnant women with suspected or confirmed influenza should receive prompt antiviral therapy, and pregnancy should not be considered a contraindication to treatment with oseltamivir or zanamivir. Oseltamivir is preferred for treatment of pregnant women because of its systemic activity. Anecdotal reports suggest postpartum women, similar to pregnant women, might be at increased risk for severe complications and death from 2009 H1N1 influenza. The transition to normal immune, cardiac, and respiratory function occurs quickly, but not immediately after delivery. Therefore, the increased risk associated with pregnancy should be considered to extend for 2 weeks postpartum regardless of the outcome of the pregnancy (including live birth, premature birth, termination of pregnancy, miscarriage, fetal death). Prompt empiric antiviral treatment is indicated for suspected or confirmed 2009 H1N1 influenza in women who are up to 2 weeks postpartum regardless of how the pregnancy ended.
Medical conditions: The following medical conditions have been associated with increased risk of complications from influenza:
- Asthma
- Neurological and neuro-developmental conditions [including disorders of the brain, spinal cord, peripheral nerve, and muscle such as cerebral palsy, epilepsy (seizure disorders), stroke, intellectual disability (mental retardation), moderate to severe developmental delay, muscular dystrophy, or spinal cord injury].
- Chronic lung disease (such as chronic obstructive pulmonary disease [COPD] and cystic fibrosis)
- Heart disease (such as congenital heart disease, congestive heart failure and coronary artery disease)
- Blood disorders (such as sickle cell disease)
- Endocrine disorders (such as diabetes mellitus)
- Kidney disorders
- Liver disorders
- Metabolic disorders (such as inherited metabolic disorders and mitochondrial disorders)
- Weakened immune system due to disease or medication (such as people with HIV or AIDS, or cancer, or those on chronic steroids)
- People younger than 19 years of age who are receiving long-term aspirin therapy
Local public health authorities might provide additional guidance about prioritizing treatment within groups at higher risk for severe infection.
Clinical Assessment
While most persons who have had confirmed or suspected 2009 H1N1 influenza have had a mild, uncomplicated self-limited respiratory illness similar to typical seasonal influenza and while persons not considered to be at increased risk of developing severe or complicated illness may not require treatment, they can be considered for antiviral treatment. Benefits of treating such patients might include a reduced duration of illness. However, based on experience with seasonal influenza treatment, patients not considered to be at increased risk of developing severe or complicated illness and who have mild, uncomplicated illness are not likely to benefit from treatment if initiated more than 48 hours after illness onset. Clinical judgment is always an essential part of treatment decisions.
People who are already recovering from influenza do not need antiviral medications for treatment. Options for close follow-up should be carefully considered. Clinicians who prefer not to treat empirically should discuss signs and symptoms of worsening illness with such patients and arrange for follow up at least by telephone.
Clinical algorithm for consideration in the assessment of persons with mild or uncomplicated influenza illness
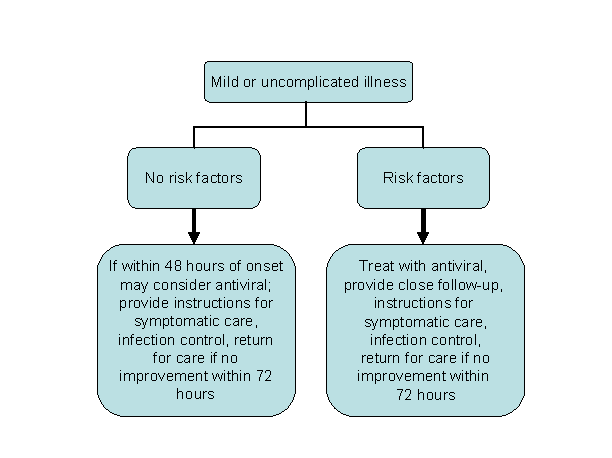
Initiation of Treatment
Treatment should be started empirically based on clinical judgment as early as possible even before definitive diagnostic test results become available, i.e., treatment should not wait for laboratory confirmation of influenza. Treatment is most effective when started in the first 48 hours of illness. As noted above, however, evidence suggests treatment may benefit patients with prolonged or severe illness even when started more than 48 hours after the onset of illness.
- Definitive testing for 2009 H1N1 requires real-time reverse transcriptase-polymerase chain reaction (rRT-PCR) or viral culture. These tests should be prioritized for persons with suspected or confirmed influenza requiring hospitalization and based on guidelines from local and state health departments.
- Rapid Influenza Diagnostic Tests (RIDTs) should not be used to rule out influenza because false negative results are common. The sensitivity of rapid tests in detecting 2009 H1N1 has ranged from 10% to 70%. Clinicians should not withhold treatment based on a negative rapid test result. Information on the use of rapid influenza diagnostic tests (RIDTs) is available online.
Methods for reducing delays in treatment initiation include:
- Inform persons at increased risk for influenza complications about the signs and symptoms of influenza and the need for early treatment after onset of symptoms.
- Ensure rapid access to telephone consultation and clinical evaluation for these patients as well as patients who report severe illness.
- Consider empiric treatment of patients at increased risk for influenza complications based on telephone contact if hospitalization is not indicated and if this will substantially reduce delay before treatment is initiated.
- Request that patients at increased risk for influenza complications contact their provider if signs or symptoms of influenza develop, obtain medication as quickly as possible if prescribed by the provider, and initiate treatment. Providers should take into account patient reliability, ability to understand the information about symptoms of influenza, and access to a pharmacy when considering ways to reduce treatment delays.
- Counsel patients about influenza antiviral benefits and adverse effects, the potential for continued susceptibility to influenza virus infection after treatment is completed (because of other circulating influenza viruses or if illness was due to another cause), and the need to again seek early access to healthcare consultation if symptoms recur.
- For persons exposed to influenza, close monitoring for symptoms and early treatment is emphasized as an alternative to chemoprophylaxis. Household or close contacts (with risk factors for influenza complications) of confirmed or suspected cases can be counseled about the early signs and symptoms of influenza, and advised to immediately contact their healthcare provider for evaluation and possible early treatment if clinical signs or symptoms develop.
Infection Control Measures for Patients Treated for Influenza
Following are recommendations to minimize exposure to infected patients. For more detailed recommendations “Antiviral Chemoprophylaxis of Exposed Individuals.”
Reducing Transmission from Infected Patients
The infectious interval for influenza is defined in the Introduction. Because some studies have shown that treatment reduces the duration and magnitude of viral shedding, treatment might also reduce infectiousness. However, symptomatic individuals who are receiving treatment should be advised that they remain potentially infectious to others while on treatment. Despite treatment with antiviral agents, patients may continue to shed influenza virus for up to four or more days after beginning therapy. Therefore, patients should maintain good cough etiquette, respiratory hygiene, and handwashing practices during the entire period on therapy to prevent the transmission of virus to close contacts.
Close contact, for the purposes of this document, is defined as having cared for or lived with a person who is a confirmed, probable, or suspected case of influenza, or having been in a setting where there was a high likelihood of contact with respiratory droplets and/or body fluids of such a person. Examples of close contact include sharing eating or drinking utensils, or any other contact between persons likely to result in exposure to respiratory droplets. Close contact typically does not include activities such as walking by an infected person or sitting across from a symptomatic patient in a waiting room or office.
Health care workers should instruct their patients in these behavioral measures. More detailed infection control measures for individuals, for preventing occupational infection, and for institutional settings are described below.
Protection of health care workers
Key recommendations to protect health care workers from being infected with influenza virus include:
- Appropriate administrative controls (e.g. healthcare personnel staying home from work when ill and triaging for identification of potentially infectious patients), respiratory and hand hygiene, personal protective equipment, and vaccination should be instituted to reduce the risk of exposure or infection for healthcare personnel and first responders.
- For more information, see "Questions and Answers about CDC’s Interim Guidance on Infection Control Measures for 2009 H1N1 Influenza in Healthcare Settings, Including Protection of Healthcare Personnel"
Additional Considerations for Treatment
Children and Over-the-Counter Cold Medications, Including Aspirin
Aspirin or aspirin-containing products (e.g. bismuth subsalicylate – PeptoBismol) should not be administered to any child or adolescent less than 19 years of age with confirmed or suspected influenza due to the risk of Reye’s syndrome. For relief of fever, other anti-pyretic medications such as acetaminophen or non-steroidal anti-inflammatory drugs are recommended. Children younger than 4 years of age should not be given over-the-counter cold medications without first speaking with a healthcare provide.
Previous Vaccination for 2009 H1N1 or Seasonal Influenza
Seasonal influenza viruses may also circulate and cause illness this 2009-2010 influenza season, possibly at the same time as the 2009 H1N1 outbreaks are occurring. The severity, amount and timing of illness that 2009 H1N1 and seasonal influenza viruses will cause are uncertain. In October 2009, inactivated and live attenuated 2009 H1N1 monovalent influenza vaccines became available in the United States. All persons in a recommended vaccination target group should be vaccinated with the 2009 H1N1 monovalent vaccine and the seasonal influenza vaccine.
The seasonal influenza vaccine is not expected to provide protection against the 2009 H1N1 influenza or vice-versa. Moreover, these vaccines are not 100% effective against infection. In addition, vaccines are not effective immediately after administration. At least 2 weeks must pass for the vaccine to take effect. Therefore, a history of vaccination does not rule out influenza. Previous vaccination is not a contraindication to antiviral drug treatment.
Treatment recommendations for vaccinated individuals should parallel those for unvaccinated persons.
Persons who are vaccinated with live attenuated influenza vaccines and who are given antiviral drugs within 48 hours before or up to two weeks after vaccination might not develop immunity and should be revaccinated.
Bacterial Co-infection
Clinicians are reminded to consider bacterial co-infections that can occur during or after influenza. Therefore, empiric treatment of community acquired pneumonia should include oseltamivir and antibacterial drugs when influenza is circulating in the community and the clinician believes that influenza may be causing or coinciding with the community-acquired pneumonia. (See Infectious Disease Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clinical Infectious Diseases 2007;44:S27–72).
Treatment of influenza when oseltamivir-resistant viruses are circulating
Oseltamivir resistance is common among seasonal influenza A (H1N1) viruses. These viruses typically remain susceptible to zanamivir, rimantadine, and amantadine. However, since April 2009, very few seasonal H1N1 viruses have circulated in the United States. Therefore, treatment, when indicated, with either oseltamivir or zanamivir is appropriate. However, if viral surveillance data indicate that oseltamivir-resistant seasonal H1N1 viruses have become more common or are associated with identified community outbreaks, zanamivir or a combination of oseltamivir and rimantadine or amantadine should be considered for use as empiric treatment for patients who might have oseltamivir-resistant seasonal human influenza A (H1N1) virus infection. National surveillance data on influenza viruses circulating in the United States is available at the Weekly Flu View. State and local health departments are also a source of viral surveillance data in some areas. Guidance on empiric treatment recommendations when multiple influenza strains are circulating is available at "CDC Issues Interim Recommendations for the Use of Influenza Antiviral Medications in the Setting of Oseltamivir Resistance among Circulating Influenza A (H1N1) Viruses, 2008-09 Influenza Season".
Antiviral Chemoprophylaxis of exposed individuals
Definitions used, whom to treat prophylactically
Infectious period
- One day before fever begins until 24 hours after fever ends.
Close contact, defined by possible modes of transmission
- Droplet exposure of mucosal surfaces (e.g. nose, moth, and eyes) by respiratory secretions from coughing or sneezing
- Contact, usually of hands, with an infectious patient or fomite (a surface that is contaminated with secretions) followed by self-inoculation of virus onto mucosal surfaces such as those of the nose, mouth, and eyes; and
- Small particle aerosols in the vicinity of the infectious individual.
Who may be considered for antiviral chemoprophylaxis
- The following persons who are a close contact of a person with suspected or confirmed 2009 H1N1 influenza during the infectious period:
- Persons at high risk for complications of influenza;
- Health care workers and emergency medical personnel;
- Pregnant women.
Whom not to treat chemoprophylactically
- Groups of healthy children or adults based on potential exposures in the community, workplace, school, camp or other settings;
- If >48 hours have elapsed since the last close contact
- The close contact did not occur during the infectious period
How to prophylax
- Antiviral drugs: oseltamivir (oral), zanamivir (inhaled)
- For detailed dosing guidelines, see tables 1 and 2 below
Consider early treatment as an alternative to chemoprophylaxis
Antiviral Drugs for Chemoprophylaxis
The neuraminidase inhibitors, oseltamivir and zanamivir, are the drugs of choice for chemoprophylaxis of 2009 H1N1 influenza-exposed children and adults in the U.S. at this time. Oseltamivir formulated as capsules or oral suspension (Tamiflu®) is FDA-approved for prophylaxis of influenza in patients 1 year and older. The FDA has issued an Emergency Use Authorization for oseltamivir prophylaxis of patients less than 1 year old (see www.cdc.gov/h1n1flu/eua/tamiflu.htm). Zanamivir formulated for oral inhalation (Relenza®) is FDA-approved for prophylaxis of influenza in patients 5 years of age and older. The duration of chemoprophylaxis is 10 days. Recommendations for prophylaxis of currently circulating 2009 H1N1 influenza with these drugs are described below as well as in Fact Sheets developed under the Emergency Use Authorizations (see Rrelenza and Tamiflu ).
Early Treatment as an Alternative to Chemoprophylaxis
Early recognition of illness and prompt initiation of treatment is emphasized as an alternative to chemoprophylaxis after a suspected exposure.
- Persons with risk factors for influenza complications who are household or close contacts of confirmed or suspected cases, and healthcare personnel who have occupational exposures, should be counseled about the early signs and symptoms of influenza, and advised to immediately contact their healthcare provider for evaluation and early treatment when indicated if clinical signs or symptoms develop.
- Healthcare providers should use clinical judgment regarding situations where early recognition of illness and treatment might be an appropriate alternative to chemoprophylaxis.
- Early recognition of illness and treatment when indicated is preferred to chemoprophylaxis for healthy vaccinated persons, including healthcare workers, after a suspected exposure.
Patients given post-exposure chemoprophylaxis should be informed that the chemoprophylaxis lowers but does not eliminate the risk of influenza and that protection stops when the medication course is stopped. Patients receiving chemoprophylaxis should be encouraged to seek medical evaluation as soon as they develop a febrile respiratory illness that might indicate influenza.
Antiviral Drug Use for Control of Outbreaks in Institutions
Use of antiviral drugs for treatment and chemoprophylaxis of influenza has been a cornerstone of the control of seasonal influenza outbreaks in nursing homes and other long-term care facilities that house large numbers of patients at higher risk for influenza complications. (See MMWR: Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008). As of October 29, 2009, no outbreaks of 2009 H1N1 have been reported in nursing homes, but at least one cluster has been reported in a long-term care facility. When 2009 H1N1 outbreaks occur within such facilities, it is recommended that ill patients be treated with oseltamivir or zanamivir and that chemoprophylaxis with either oseltamivir or zanamivir be started as early as possible to reduce the spread of the virus as is recommended for seasonal influenza outbreaks in such settings. Additional guidance for infection control measures in long-term care facilities can be found at: Using Antiviral Medications to Control Influenza Outbreaks in Institutions.
In addition to use in nursing homes, antiviral chemoprophylaxis also can be considered for controlling influenza outbreaks in other closed or semi-closed settings (e.g., correctional facilities, or other settings in which persons live in close proximity) where large numbers of persons at higher risk for influenza complications are housed. For more information about influenza outbreaks in facilities see:
- Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008
- Seasonal Influenza in Adults and Children—Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management: Clinical Practice Guidelines of the Infectious Diseases Society of America
- Interim Guidance for Correctional and Detention Facilities on Novel Influenza A (H1N1) Virus
- Interim Guidance for Emergency Shelters on the Novel Influenza A (H1N1) Virus
Outbreaks of influenza in schools, camps, workplaces and other group settings should not be managed by providing chemoprophylaxis to all persons potentially exposed to influenza viruses. The healthy populations typically present in these settings should be educated about the signs and symptoms of influenza, and urged to consult their healthcare provider if severe illness develops. Post-exposure chemoprophylaxis can be considered for those who meet the above criteria for exposure and who have a medical condition or are of an age that confers a higher risk for influenza complications. An emphasis on early evaluation and treatment, as described above, is an alternative. Persons in these settings also should be educated about hygiene and infection control measures that can reduce transmission of influenza viruses.
Specific Regimens for Treatment and Chemoprophylaxis of 2009 H1N1 Influenza A
Treatment and Chemoprophylaxis Regimens for Adults and Children by Age and Weight
Antiviral Prescription and Dispensing Considerations
Alternatives to Tamiflu® Oral Suspension for Pediatric Patients
Even though commercially-manufactured Tamiflu® Oral Suspension (12 mg/mL) is the preferred product for patients who have difficulty swallowing capsules or where lower doses are needed, this product may not be locally available.
For patients who are less than one year old, there is one alternative:
- a suspension compounded by a retail pharmacy (FDA Drug Safety Information: Emergency Compounding of an Oral Suspension from Tamiflu 75 mg Capsules (Final Concentration 15 mg/mL)
For children who are at least one year old there are two alternatives:
- a suspension compounded by a retail pharmacy (same link as above)
- 30mg, 45mg, or 75 mg capsules, which may be mixed into a sweetened liquid by a caregiver if the child cannot swallow capsules ("Opening and Mixing Tamiflu Capsules with Liquids if Child Cannot Swallow Capsules"
Compounding Oseltamivir Suspension – Potential Dosing Errors
The commercially-manufactured Tamiflu® Oral Suspension concentration is 12 mg/mL; the compounded suspension concentration is 15 mg/mL.
When prescribing Tamiflu® Oral Suspension, prescribers should specify the concentration if prescribing in mL or teaspoons, or they should prescribe the dose in milligrams (mg).
Additional information can be found at:
- H1N1 Flu: Resources for Pharmacists
- Institute for Safe Medication Practices Safety Alert: Tamiflu® Oral Suspension Shortage Contributing to Dosing Errors
Commercial Tamiflu® Oral Suspension – Potential Dosing Device Errors
Pharmacists with access to Tamiflu® Oral Suspension should be aware that an oral dosing dispenser with 30 mg, 45 mg, and 60 mg graduations is provided in the manufacturer’s packaging, rather than graduations in milliliters (mL) or teaspoons (tsp). There have been cases where the units of measure on the prescription dosing instructions (mL, tsp) do not match the units on the dosing device (mg), which can lead to confusion and dosing errors. When dispensing commercially-manufactured Tamiflu® Oral Suspension, pharmacists should ensure the units of measure on the dosing instructions match the dosing device provided. If dosing instructions specify administration using mL or tsp the device included in the Tamiflu® product package should be removed and replaced with an appropriate measuring device.
When dispensing commercially-manufactured Tamiflu® Oral Suspension, please ensure that the units of measure on the dosing instructions match the dosing device provided. For children younger than 1 year of age, the oral dosing dispenser that is included in the product package should always be removed and replaced with an appropriate measuring device as it is not designed for measuring doses
Additional information can be found at:
- H1N1 Flu: Resources for Pharmacists
- FDA Public Health Alert: Potential Medication Errors with Tamiflu for Oral Suspension
Adverse Events and Contraindications
Oseltamivir and zanamivir are generally well-tolerated among FDA-approved age groups. Nausea and vomiting were reported more frequently among adults receiving oseltamivir for treatment (nausea without vomiting, approximately 10%; vomiting, approximately 9%) than among persons receiving placebo (nausea without vomiting, approximately 6%; vomiting, approximately 3%). Among children treated with oseltamivir, 14% had vomiting, compared with 8.5% of placebo recipients. Oseltamivir suspension is formulated with sorbitol, which may be associated with diarrhea and abdominal pain in patients who are fructose-intolerant. Retrospective safety data on oseltamivir treatment of seasonal influenza in children younger than 1 year old is limited and suggest that severe adverse events are rare. Prospective data continue to be collected on safety and efficacy of oseltamivir in this age group. Allergic reactions (rash, swelling of the face or tongue, anaphylaxis) have been reported in clinical practice from both oseltamivir and zanamivir.
Zanamivir, an inhaled medication, can induce bronchospasm and is not recommended for treatment for patients with an underlying increased risk of bronchospasm. Zanamivir should only be used as directed in the prescribing information by using the Diskhaler device provided with the drug product. The commercial zanamivir formulation (Relenza® Inhalation Powder) is a mixture of zanamivir active drug substance and lactose drug carrier. This formulation is not designed or intended to be used in any nebulizer or mechanical ventilator as there is a risk that the lactose sugar can obstruct proper functioning of mechanical ventilator equipment. Although there are published and unpublished reports of zanamivir being used via nebulizer and mask in clinical trials, the currently available commercial formulation is not designed or intended to be administered by nebulization.
Rarely, transient neuropsychiatric events (self-injury or delirium) have been reported in postmarketing surveillance among persons taking oseltamivir and zanamivir. The majority of reports were among children and adolescents living in Japan. Because influenza infection itself can be associated with a variety of neurologic and behavioral symptoms, including seizures, delirium, and hallucinations, whether the neuraminidase inhibitors are directly responsible for these neuropsychiatric effects is unclear. To date, retrospective analyses conducted by Roche, the manufacturer of oseltamivir, have not found evidence for an increased risk of neuropsychiatric events after oseltamivir use. Until additional data are available, FDA advises that persons receiving neuraminidase inhibitors be monitored for abnormal behavior. For additional information please refer to the FDA MedWatch Safety Alert: Tamiflu®.
Data on the safety of peramivir IV are limited and are summarized in the EUA Factsheet for Health Care Professionals. After a single dose of 200 mg or 400 mg peramivir IV, adverse clinical events and laboratory abnormalities were no more frequent than placebo. Among hospitalized patients treated with 5 days of peramivir IV, 200 mg or 400 mg, overall adverse events were recorded in 54% of patients compared with 41% of patients treated with oseltamivir 75 mg orally twice daily. Serious adverse events were noted in 4% of patients receiving 200 mg daily, 17% of patients receiving 400 mg, and 9% of patients receiving oseltamivir. Diarrhea and psychiatric events were more common among peramivir recipients than oseltamivir recipients. In terms of laboratory parameters, clinicians should monitor blood counts, electrolytes, renal function, urinalysis, and hepatic profile as recommended in the EUA. Given the limited safety data on peramivir, mandatory reporting requirements are part of the conditions of the EUA. Health care providers (or designee) must report adverse events and all medication errors associated with peramivir to FDA’s MedWatch program within 7 calendar days from the onset of the adverse event, including follow-up as requested.
Reporting Adverse Events
Health care professionals should report all serious adverse events (SAE) after antiviral medication use promptly to MedWatch, the FDA’s adverse event reporting program for medications.
For More Information
For further information about influenza and antiviral medications, including contraindications and adverse effects, please see the following:
- Questions&Answers: Antiviral Drugs, 2009-2010 Flu Season
- Antiviral Agents for Seasonal Influenza: Side Effects and Adverse Reactions.
- Harper SA, Bradley JS, Englund JA, et al. Infectious Diseases Society of America Guidelines. Seasonal Influenza in Adults and Children—Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management: Clinical Practice Guidelines of the Infectious Diseases Society of America.
- Jain, S et al. Hospitalized Patients with 2009 H1N1 Influenza in the United States, April-June 2009. N Engl J Med 2009;361.
- Jamieson DJ, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009;374:451-8.
- CDC. Intensive-Care Patients With Severe Novel Influenza A (H1N1) Virus Infection --- Michigan, June 2009. MMWR Morb Mortal Wkly Rep 2009:58:749-52.
- Rasmussen SA. Pandemic Influenza and Pregnant Women: Summary of a Meeting of Experts. Am J Public Health 2009; 99 (S2): S248-S254.
Links to non-federal organizations are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the federal government, and none should be inferred. CDC is not responsible for the content of the individual organization Web pages found at these links.
Get email updates
To receive weekly email updates about this site, enter your email address:
Contact Us:
- Centers for Disease Control and Prevention
1600 Clifton Rd
Atlanta, GA 30333 - 800-CDC-INFO
(800-232-4636)
TTY: (888) 232-6348 - Contact CDC-INFO