CDC H1N1 Flu Update: U.S. Human Cases of H1N1 Flu Infection
Novel Influenza A (H1N1) Cases by HHS Joint Field Office Coordination Groups
May 12, 2009, 11:00 AM ET
3009 Confirmed Cases in 45 States
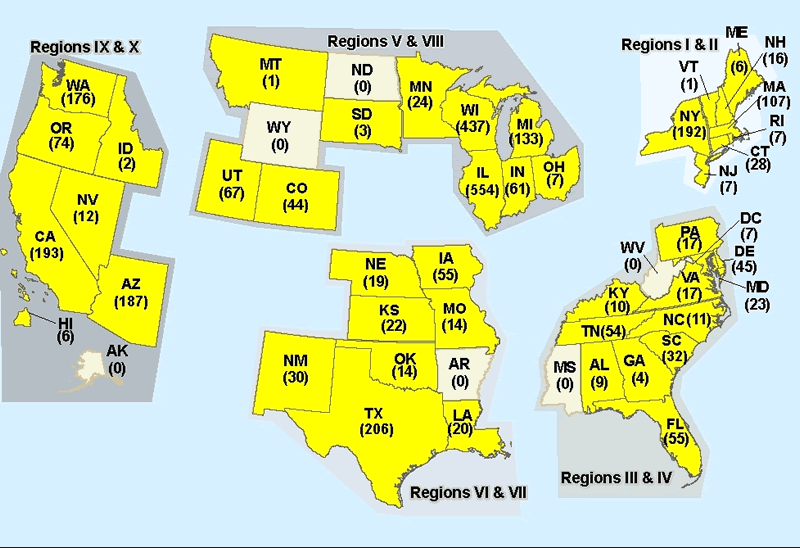
| States* | Laboratory confirmed cases |
Deaths | |
|---|---|---|---|
| Alabama | 9 | ||
| Arizona | 187 | ||
| California | 193 | ||
| Colorado | 44 | ||
| Connecticut | 28 | ||
| Delaware | 45 | ||
| Florida | 55 | ||
| Georgia | 4 | ||
| Hawaii | 6 | ||
| Idaho | 2 | ||
| Illinois | 554 | ||
| Indiana | 61 | ||
| Iowa | 55 | ||
| Kansas | 22 | ||
| Kentucky** | 10 | ||
| Louisiana | 20 | ||
| Maine | 6 | ||
| Maryland | 23 | ||
| Massachusetts | 107 | ||
| Michigan | 133 | ||
| Minnesota | 24 | ||
| Missouri | 14 | ||
| Montana | 1 | ||
| Nebraska | 19 | ||
| Nevada | 12 | ||
| New Hampshire | 16 | ||
| New Jersey | 7 | ||
| New Mexico | 30 | ||
| New York | 192 | ||
| North Carolina | 11 | ||
| Ohio | 7 | ||
| Oklahoma | 14 | ||
| Oregon | 74 | ||
| Pennsylvania | 17 | ||
| Rhode Island | 7 | ||
| South Carolina | 32 | ||
| South Dakota | 3 |
||
| Tennessee | 54 |
||
| Texas | 206 |
2 | |
| Utah | 67 | ||
| Vermont | 1 |
||
| Virginia | 17 |
||
| Washington | 176 | 1 | |
| Washington, D.C. | 7 | ||
| Wisconsin | 437 |
||
| TOTAL*(45) | 3009 cases | 3 deaths | |
International Human Cases of Swine Flu Infection *includes the District of Columbia **one case is resident of KY but currently hospitalized in GA. NOTE: Because of daily reporting deadlines, the state totals reported by CDC may not always be consistent with those reported by state health departments. If there is a discrepancy between these two counts, data from the state health departments should be used as the most accurate number. |
|||
A New Virus Emerges
Novel influenza A (H1N1) is a new flu virus of swine origin that was first detected in April, 2009. The virus is infecting people and is spreading from person-to-person, and has sparked a growing outbreak of illness in the United States with an increasing number of cases being reported internationally as well.
CDC anticipates that there will be more cases, more hospitalizations and more deaths associated with this new virus in the coming days and weeks because the population has little to no immunity against it. Novel influenza A (H1N1) activity is now being detected in two of CDC’s routine influenza surveillance systems as reported in the May 8, 2009 FluView. FluView is a weekly report that tracks U.S. influenza activity through multiple systems across five categories.
The May 8 FluView found that the number of people visiting their doctors with influenza-like-illness is higher than expected in the United States for this time of year. Second, laboratory data shows that regular seasonal influenza A (H1N1), (H3N2) and influenza B viruses are still circulating in the United States, but novel influenza A (H1N1) and “unsubtypable”* viruses now account for a significant number of the viruses detected in the United States.
It’s thought that novel influenza A (H1N1) flu spreads in the same way that regular seasonal influenza viruses spread; mainly through the coughs and sneezes of people who are sick with the virus.
CDC continues to take aggressive action to respond to the outbreak. CDC’s response goals are to reduce the spread and severity of illness, and to provide information to help health care providers, public health officials and the public address the challenges posed by this new public health threat.
Increased Testing
CDC has developed a PCR diagnostic test kit to detect this novel H1N1 virus and has now distributed test kits to all states in the U.S. and the District of Columbia and Puerto Rico. The test kits are being shipped internationally as well. This will allow states and other countries to test for this new virus. This increase in testing will likely result in an increase in the number of confirmed cases of illness reported. This, combined with ongoing monitoring through Flu View should provide a fuller picture of the burden of disease in the United States over time.
CDC is issuing updated interim guidance daily in response to the rapidly evolving situation.
Clinician Guidance
CDC has issued interim guidance for clinicians on identifying and caring for patients with novel H1N1, in addition to providing interim guidance on the use of antiviral drugs. Influenza antiviral drugs are prescription medicines (pills, liquid or an inhaler) with activity against influenza viruses, including novel influenza H1N1 viruses. The priority use for influenza antiviral drugs during this outbreak is to treat severe influenza illness, including people who are hospitalized or sick people who are considered at high risk of serious influenza-related complications.
Public Guidance
In addition, CDC has provided guidance for the public on what to do if they become sick with flu-like symptoms, including infection with novel H1N1. CDC also has issued instructions on taking care of a sick person at home. Novel H1N1 infection has been reported to cause a wide range of symptoms, including fever, cough, sore throat, body aches, headache, chills and fatigue. In addition, a significant number of people also have reported nausea, vomiting or diarrhea. Everyone should take everyday preventive actions to stop the spread of germs, including frequent hand washing and people who are sick should stay home and avoid contact with others in order to limit further spread of the disease.
*Unsubtypable viruses are viruses that through normal testing cannot be subtyped as regularly occurring human seasonal influenza viruses. In the context of the current outbreak, it’s likely that most of these unsubtypable viruses are novel H1N1.
More on the Situation
- Links to non-federal organizations are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the federal government, and none should be inferred. CDC is not responsible for the content of the individual organization Web pages found at these links.

