Error processing SSI file

NOTE:This page is not intended as a stand-alone Web document and is intended to serve merely as a Section 508-accessible version of the PowerPoint presentation "2009 H1N1: Overview of a Pandemic, April 2009 - August 2010."
2009 H1N1: Overview of a Pandemic
2009 H1N1 Accomplishments (Part 1 of 3)
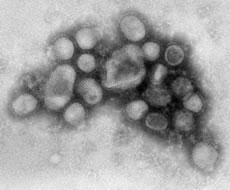
- CDC’s Influenza Division laboratories in Atlanta were the first in the world to identify the new 2009 H1N1 virus strain

- CDC’s testing revealed that 2009 H1N1 was a new (novel) influenza virus strain that had not been previously detected in humans or animals
- The new 2009 H1N1 virus was originally referred to as “swine flu” because initial laboratory tests at CDC showed that six of the virus’s eight genes were most closely related to influenza viruses that normally occur in pigs in North America, and the remaining two genes were most closely related to influenza viruses circulating in Eurasian pigs

- CDC uploaded the complete gene sequences of the 2009 H1N1 Virus to a publicly-accessible international influenza database, which enabled scientists around the world to use the sequences for public health research and for comparison against influenza viruses collected elsewhere
- CDC’s subject matter experts worked around the clock to create guidance documents for the general public, business, schools, child care programs, at-risk populations, clinicians, travelers, laboratory specialists, etc

- Many of these documents were revised several times as the outbreak developed and as new information became available
- Once the 2009 H1N1 outbreak was detected, CDC enhanced its existing surveillance systems and added several new surveillance systems to better track the spread, disease characteristics and burden of the new virus
- One of the new surveillance systems was the Aggregate Hospitalizations and Deaths Reporting Activity (AHDRA), which was a Web-based system used to track state reports of laboratory-confirmed and syndromic flu-related hospitalizations and deaths

- Because experts acknowledged that individual reports of hospitalizations and deaths likely represented an undercount of what was occurring in actuality, CDC also developed a method to estimate 2009 H1N1 cases, hospitalizations and deaths in the United States. The methodology to derive these estimates was based in part on extrapolation from AHDRA data
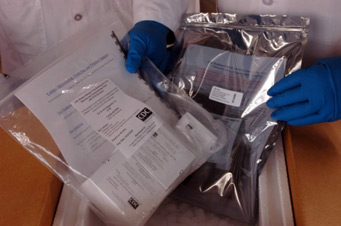
- CDC laboratory experts quickly developed primers and probes that could be incorporated into previously manufactured tests to identify the 2009 H1N1 Virus in respiratory (nose, throat and lung) samples collected from patients with 2009 H1N1 illness

- Expedited FDA approval of an emergency use authorization (EUA) for these 2009 H1N1 diagnostic tests occurred on April 28, 2009, less than two weeks after identification of the 2009 H1N1 strain
- On May 1, 2009, CDC test kits began shipping to domestic and international public health laboratories. As of August 2010, 2,344 RT-PCR test kits have been shipped to 442 labs in 145 countries and 305 WHO HI test kits have been shipped to 207 labs in 99 countries

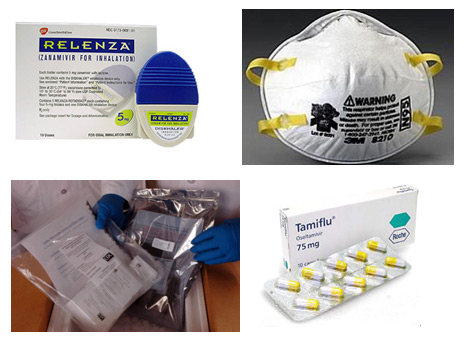
- In response to Secretary of Heath and Human Services (HHS) Kathleen Sebelius’ declaration of a public health emergency involving 2009 H1N1 flu on April 26, 2009, the FDA authorized the emergency use of important medical products under certain circumstances
- FDA issued EUAs on the five medical products CDC requested, which allowed for the emergency use of antiviral products (Relenza and Tamiflu), certain types/models of N95 respirators, the rRT-PCR Swine Flu Panel diagnostic test, and the rRT-PCR Flu Panel (NPS, NS, TS, NPS/TS, NA) diagnostic test
- One of the most important laboratory activities performed at CDC was the selection and development of a candidate vaccine virus for use in the 2009 H1N1 vaccine
- CDC’s laboratories took the first step of picking a candidate vaccine virus, which involves choosing a 2009 H1N1 virus that can be grown in mass quantities in chicken eggs

- Once the virus is grown in mass quantities, the parts of the virus that are important for forming an immune response to the vaccine (the virus antigens), are purified to make the vaccine
- CDC pursued several scientific methods to create a high-yield vaccine candidate virus for the 2009 H1N1 vaccine, and after consultation with WHO and the Food and Drug Administration (FDA), the A/California/7/2009 virus – a virus isolated at CDC – was chosen to be the vaccine candidate strain for making the 2009 H1N1 vaccine
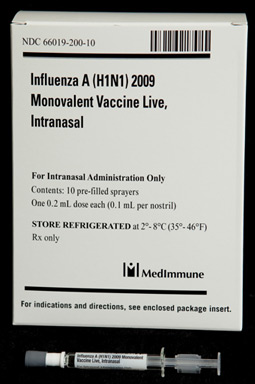
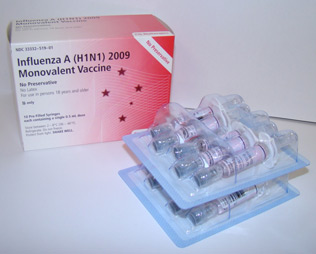
- After the virus was ready on April 27, 2009, CDC began sending it to vaccine manufacturing companies to begin the process of mass producing a vaccine to protect people against 2009 H1N1

- The 2009 H1N1 vaccine was made using the same egg-based manufacturing process used for making the seasonal flu vaccine. These techniques have been used to reliably produce safe flu vaccines for decades
- The vaccine strain grew slowly in chicken eggs, resulting in delays in availability of initial lots of the vaccine. This led to some frustration on the part of the public during the early part of the fall wave of the pandemic, when vaccine supplies were limited
- Despite the delays, the 2009 H1N1 vaccine was made available in near-record time, considering current technological limitations

Next: 2009 H1N1 Accomplishments (continued) >
Get email updates
To receive weekly email updates about this site, enter your email address:
Contact Us:
- Centers for Disease Control and Prevention
1600 Clifton Rd
Atlanta, GA 30333 - 800-CDC-INFO
(800-232-4636)
TTY: (888) 232-6348 - Contact CDC-INFO