Multistate Outbreak of Fungal Meningitis and Other Infections
October 30, 2015 further updates to the case counts are not anticipated at this time.
On October 30, 2015, CDC updated its web resources for patients and clinicians. Patients affected by tainted steroid injections from the New England Compounding Center continue to receive treatment for their infections and clinicians should continue to monitor patient recovery. All relevant materials for patients and clinicians concerning the multistate outbreak of fungal meningitis and other infections are located on this page.
Highlights
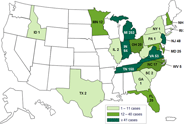
In September 2012, the Centers for Disease Control and Prevention (CDC), in collaboration with state and local health departments and the Food and Drug Administration (FDA), began investigating a multistate outbreak of fungal meningitis and other infections among patients who received contaminated preservative-free MPA steroid injections from the New England Compounding Center in Framingham, Massachusetts.
The investigation includes fungal meningitis (a form of meningitis that is not contagious), localized spinal or paraspinal infections, such as epidural abscess and arachnoiditis, and infections associated with injections in a peripheral joint space, such as a knee, shoulder, or ankle.
The predominant fungus identified in patients is Exserohilum rostratum. One patient, the index case, had a laboratory-confirmed Aspergillus fumigatus infection. These fungi are common in the environment, however fungal infections are not transmitted from person to person.
On October 6, 2012 NECC voluntarily recalled all products compounded at and distributed from its facility in Framingham, Massachusetts.
On October 31, 2012 The U.S. Food and Drug Administration announced that Ameridose, LLC, based in Westborough, Mass., voluntarily recalled all of its unexpired products in circulation.
- CDC Information About Voluntary Recall of All Ameridose Medical Products
- FDA Information on the recall of all Ameridose products.
November 1, 2012 Health Alert Network (HAN): Contamination Identified in Additional Medical Products from New England Compounding Center
November 1, 2012 Health Alert Network (HAN): Voluntary Recall of All Ameridose Medical Products
December 3, 2012 Health Alert Network (HAN): Additional Contamination Identified in Medical Products from New England Compounding Center
Timeline of Recall
On September 26, 2012, NECC voluntarily recalled three lots of preservative-free MPA associated with the multistate outbreak of fungal meningitis and other infections.
On October 6, NECC expanded its recall to include all products in circulation that were distributed from its facility in Framingham, Massachusetts.
On October 11, 2012 FDA released a MedWatch Alert stating that samples of injectable betamethasone and cardioplegia solution tested positive for bacterial contamination. The FDA and CDC laboratories have identified bacteria and/or fungi present in NECC-supplied preservative-free injectable betamethasone, preservative-free triamcinolone, and cardioplegia solution (specific lots listed below).
FDA released a MedWatch Safety Alert on October 15 stating that the sterility of any injectable drugs, including ophthalmic drugs that are injectable or used in conjunction with eye surgery, and cardioplegia solutions produced by NECC is of significant concern.
Although CDC has received reports of illness in patients who have received the medications listed in the table below, including some patients who had evidence of meningeal inflammation, CDC and public health officials have no reports of laboratory-confirmed bacterial or fungal meningitis, spinal, or paraspinal infections caused by these products. [This information was accurate as of [FinalDateReviewed]]
The available epidemiological and laboratory data do not, support evidence of an outbreak of infections linked to usage of non-methylprednisolone NECC products.
If you have taken or used any medication from NECC and feel sick, you should seek medical attention. Any infections potentially related to NECC products should be reported to FDA’s MedWatch and to your state health department.
Laboratory Testing and Results
CDC and FDA identified bacterial and/or fungal contamination in unopened vials of betamethasone, cardioplegia, and triamcinolone solutions distributed and recalled from NECC. These include bacteria known as Bacillus, and fungal species including Aspergillus tubingensis, Aspergillus fumigatus, Cladosporium species, and Penicillium species. Although rare, some of the identified Bacillus species can cause disease in humans. Some of the fungal organisms identified, particularly Aspergillus fumigatus, are also known to cause disease in humans. It is not known how product contamination with these organisms could affect patients clinically. See CDC’s Advice for Clinicians below.
CDC and FDA Laboratory-Confirmed Organisms from Product Samples
| Laboratory-Confirmed Organisms from Product Samples Associated with NECC Recalled Lots of Betamethasone, Cardioplegia, and Triamcinolone Solutions | ||
|---|---|---|
| Medication | Lot Number | Microbial Contamination |
| Betamethasone
6 mg/mL injectable – 5 mL per vial |
08202012@141 | Paenibacillus pabuli/amolyticus; Bacillus idriensis; Bacillus flexus; Bacillus simplex; Lysinibacillus sp., Bacillus niacini, Kocuria rosea, Bacillus lentus |
| Betamethasone
6 mg/mL injectable – 5 mL per vial |
07032012@22 | Bacillus niabensis; Bacillus circulans |
| Betamethasone
12 mg/mL injectable – 5 mL per vial |
07302012@52 | Bacillus lentus, Bacillus circulans, Bacillus niabensis, Paenibacillus barengoltzii/timonensis |
| Betamethasone
6mg/mL injectable – 5 mL per vial |
08202012@44 | Bacillus lentus, Bacillus firmus, Bacillus pumilus |
| Betamethasone
6 mg/mL injectable – 5 mL per vial |
08152012@84 | Penicillium sp., Cladosporium sp. |
| Triamcinolone
40mg/mL injectable – 1 mL per vial |
06062012@6 | Bacillus lentus, Bacillus circulans, Bacillus niabensis, Bacillus nealsonii, Bacillus subtilis group, Bacillus firmus |
| Triamcinolone
40 mg/mL injectable – 2 mL per vial |
08172012@60 | Aspergillus tubingensis, Penicillium sp. |
| Triamcinolone
40mg/mL injectable – 10 mL per vial |
08242012@2 | Aspergillus fumigatus |
| Cardioplegia solution 265.5 mL per bag |
09242012@55 | Bacillus halmapalus/horikoshii, Brevibacillus choshinensis |
Advice to Clinicians
FDA released a MedWatch Safety Alert on October 15 stating that the sterility of any injectable drugs, including ophthalmic drugs that are injectable or used in conjunction with eye surgery, and cardioplegia solutions produced by NECC is of significant concern. FDA advised healthcare providers to follow-up with patients who were administered any of these products purchased from or distributed by NECC on or after May 21, 2012.
CDC recommends that clinicians remain vigilant for the possibility that infections may have resulted from injection of NECC products, and that routine laboratory and microbiologic tests, including bacterial and fungal cultures, should be obtained as necessary by treating clinicians.
There has been no prior regular surveillance for adverse events following epidural steroid injections; however, infection is a known, although likely rare, risk that has been documented in the medical literature. Although CDC has received reports of illness in patients who have received the medications listed in the table above, including some patients who had evidence of meningeal inflammation, CDC and public health officials have no reports of laboratory-confirmed bacterial or fungal meningitis, spinal, or paraspinal infections caused by these products. The available epidemiological and laboratory data do not, at this time, support evidence of an outbreak of infections linked to usage of non-methylprednisolone NECC products.
However, because it is possible that some of the organisms listed in the table above can cause human disease, clinicians should continue to include bacterial and/or fungal infection in the differential diagnosis when evaluating symptomatic patients who were exposed to these medications, including consideration of empiric anti-bacterial and/or antifungal therapy.
Consultation with an infectious disease specialist is strongly encouraged to help make diagnosis and treatment decisions in these cases. If the evaluation of these patients is suggestive of fungal infection, please consult existing CDC treatment guidance associated with this outbreak.
Physicians should continue to report infections potentially related to NECC products to FDA’s MedWatch and to state health departments.
Advice to Patients
If you have taken or used any medication from NECC and feel sick, you should seek medical attention. Any infections potentially related to NECC products should be reported to FDA’s MedWatch and to your state health department.
CDC Clinician Resources
- CDC Health Alert Network (HAN): Update: Multistate Outbreak of Fungal Infections among Persons Who Received Injections with Contaminated Medication, December 20, 2012
- CDC Health Alert Network (HAN): Update: Additional Contamination Identified in Medical Products from New England Compounding Center December 3, 2012
- CDC Health Alert Network (HAN): Contamination Identified in Additional Medical Products from New England Compounding Center, November 1, 2012
- Information About Voluntary Recall of All Ameridose Medical Products
FDA Clinician Resources
- Update on NECC Products: Samples of injectable betamethasone and cardioplegia solution test positive for bacterial contamination
- FDA reports conditions observed at New England Compounding Center facility (FDA Press Release: Oct. 26, 2012)
- Patient Notification Letter [PDF – 1 page]
- List of customer names and addresses, organized by state [PDF – 73 pages]
- List organized alphabetically and by product ,quantities and shipping date [PDF – 345 pages]
- FDA Form 483 for New England Compounding Center [PDF – 8 pages]
- Meningitis Outbreak: Voriconazole and Liposomal Amphotericin B Availability Information