Integrated Viral Hepatitis Surveillance and Prevention Funding for Health Departments (IVHSP): Frequently Asked Questions [Archived]
CDC-RFA-PS21-2103
The content on this webpage is available for historical purposes. CDC is no longer reviewing or updating this content.
Index of Questions
General Information
What is the importance of this combined Notice of Funding Opportunity (NOFO) for the states?
This combined NOFO was developed to help states
- promote integration of prevention and surveillance activities;
- streamline application, reporting requirements, and project management;
- establish national viral hepatitis surveillance capacity;
- provide a mechanism to enhance activities, information exchange, and technical support across all jurisdictions, CDC, and stakeholders; and
- promote viral hepatitis elimination planning and enhance use of data to prioritize and evaluate viral hepatitis elimination initiatives.
How will technical assistance be provided to recipients (all components)?
Please refer to the NOFO (CDC Program Support to Recipients) for the description of technical assistance that CDC will provide to recipients. While all assistance listed is relevant for all activities, the bolded items indicate either surveillance-specific activities or activities that will require surveillance data. CDC will
- collaborate to ensure coordination and implementation of strategies to support the implementation of comprehensive viral hepatitis surveillance and prevention activities;
- provide guidance and coordination to funded organizations to improve the quality and effectiveness of work plans, evaluation strategies, products and services, and collaborative activities with other organizations;
- collaborate to ensure coordination and provide policy and program information for rapid dissemination and implementation;
- work with recipients to identify and address capacity building assistance and technical assistance (TA) needs that are essential to the success of the project;
- provide access to training and TA that will strengthen staff capacity relevant to all required strategies and activities of the program;
- provide guidance to the recipient and set standards on data collection, use, and submission requirements;
- provide guidance and technical assistance in implementing the Hepatitis Message Mapping Guide (MMG) and facilitate collaborations with the Center for Surveillance, Epidemiology, and Laboratory Services (CSELS) to transition to HL7-based case reporting to CDC through the National Notifiable Diseases Surveillance System (NNDSS);
- provide technical advice in the development of systems to implement and advance CDC policies, initiatives, and programs;
- collaborate to ensure coordination and implementation of TA to state and local health department viral hepatitis program staff;
- collaborate in assessing progress toward meeting goals/outcomes and in establishing measurement and accountability systems for documenting outcomes, such as increased performance improvements and best or promising practices);
- provide guidance and coordinate with the recipient to improve the quality and effectiveness of the proposed program (this may include revision of the work plan, evaluation strategy, and products and services);
- foster and support ongoing opportunities for networking, communication, coordination, and collaboration;
- provide consultation in planning, operating, analyzing, and evaluating viral hepatitis programs, including viral hepatitis elimination planning, and CDC special initiatives (e.g., program integration, viral hepatitis elimination, and program evaluation activities);
- monitor recipient program performance using multiple approaches, such as standardized review of performance, recipient feedback, and other data reports to support program development, implementation, evaluation, and improvement;
- provide support and facilitate program collaboration with other CDC programs and HHS offices to enhance and improve integration of services;
- assist in assessing program operations and evaluating overall effectiveness of programs;
- provide capacity building assistance where identified or as needed;
- collect and disseminate information, best practices, lessons learned, and evaluation results (e.g., through conferences, guidance, material development, webinars, data sharing publications, other social media, participation in meetings, committees, and working groups related to the cooperative agreement); and
- provide requirements and expectations for standardized and other data reporting and support monitoring and evaluation activities.
What is the importance of collaboration for this NOFO (all components)?
Collaboration can lead to improved stakeholder recommendations for the jurisdiction; improved efficiency, quality, and completeness of surveillance and access to data and information; and improved engagement, and communication and cross-collaboration among disparate agencies, policymakers, providers who care for PWIDs, and members of other focus populations identified by the jurisdiction. Ultimately, collaborations should culminate in development and implementation of a data-driven viral hepatitis elimination plan for the jurisdiction accompanied by full stakeholder buy-in and engagement.
How will jurisdictions be expected to carry out the full requirements of the NOFO during the current COVID-19 pandemic?
Recipients will receive support and technical assistance from CDC to carry out the core components of the NOFO. Additionally, CDC encourages adaptive methods for accomplishing objectives, to include virtual meetings with stakeholders, using telehealth services, and employing other models for socially distanced testing and treatment.
What does “contingent on funding” mean?
“Contingent on funding” means that there is an expectation that an indicated activity will be conducted if funding is available. At this time, we do not know if funding will be available for activities currently listed as “contingent on funding.”
Are there any restrictions on funding?
If jurisdictions wish to support an SSP, they must show evidence that they have submitted a request for Determination of Need (DON). Funding cannot be allocated without an approved DON. Additionally, federal funds from this award may not be used to purchase
- medications/drugs for treatment or care;
- hepatitis A, hepatitis B, or any other vaccines;
- sterile injection needles or syringes for drug injection;
- other devices solely used for illegal drug injection (e.g., cookers); or
The NOFO doesn’t specify award floors or ceilings for the three components. Can you give any recommendation of the maximum that a jurisdiction can request in these categories? Can you provide guidance for the awards you have in mind for the activities that are contingent on funding?
The estimated average year 1 funding amounts listed in the PS21-2103 funding opportunity announcement for Components 1, 2, and 3 are neither funding floors nor funding ceilings. The estimated average awards for Component 1 and Component 2 are meant to guide applicants in the development of their budgets for the elements that are not contingent on funding within that specific Component. While applicants are encouraged to keep estimated funding amounts in mind when developing their budgets, they are able to submit budgets consistent with their actual needs. The estimated average year 1 funding amount for Component 3 is provided to guide applicants in the development of their budget for element 3.1; similarly, it is not a floor nor a ceiling.
Within the same Component, budget details for elements “contingent on funding” should be detailed separately from elements not contingent on funding. No estimated average funding amounts for year 1 are provided for Component 1 and 2 elements that are “contingent on funding;” therefore, applicants should request what funds they believe are necessary to complete these elements (e.g. 1.3, 2.2, and 2.3).
Can this funding support the procurement of hepatitis C test kits for high impact sites?
Yes. Funding can be used to purchase test kits under Components 2 and 3.
Regarding page limit of the application, the instructions state that the Narrative (which includes the Background, Approach, Purpose, Outcomes, Strategies & Activities, Collaborations, Target Populations & Health Disparities, Evaluation and Performance Measurement Plan including Data Management Plan, Organizational Capacity, and Work Plan) should be no longer than 20 pages. Is it 20 pages in total, or 20 pages for each of the three components?
For reference, please see page 74 of the NOFO. Applicants are allowed 10 pages for the “base.” The base can cover all subsections of the project description that the components share with each other: Background, Target Population, Collaboration, Health Disparities, Organizational Capacity (include organizational capacity for each component). Applicants are allowed up to 10 additional pages for each component to describe specific project narrative subsections (Approach, Evaluation and Performance Plan, and Work Plan).
Applicants seeking funding for Components 1 and 2 can submit:
- Base (10 pages) +
- Component 1-specific narrative (10 pages) +
- Component 2-specific narrative (10 pages) =
- TOTAL 30 pages
Applicants seeking funding for Components 1, 2, and 3 can submit:
- Base (10 pages) +
- Component 1-specific narrative (10 pages) +
- Component 2-specific narrative (10 pages) +
- Component 3-specific narrative (10 pages) =
- TOTAL 40 pages
Are there going to be standard workplan, budget templates, and narratives for this grant put forth by the Program?
The Division is currently consulting with the Office of Grant Services regarding providing examples of Resource Documents. Once confirmed, updates will be available and shared via the Viral Hepatitis’s NOFO webpage.
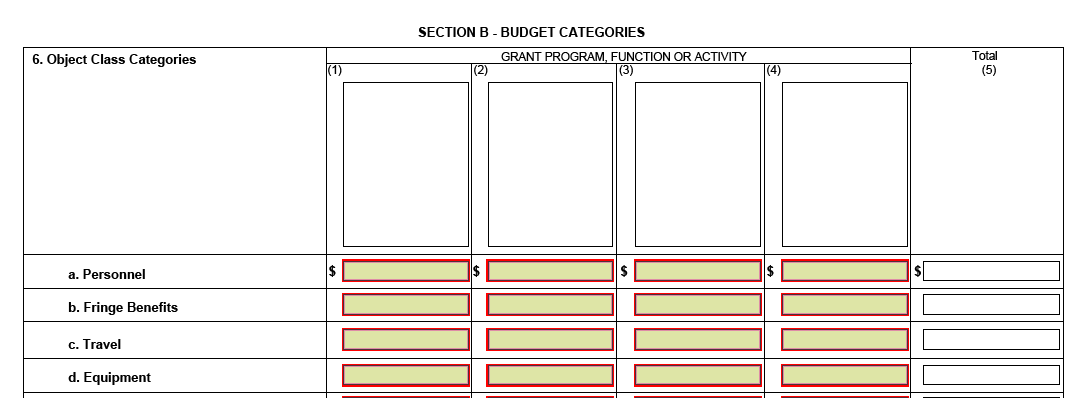
Are there three SF 424As required as well as three budgets?
No. Only one form SF424A will be required for submission. Section B of the SF424A will accommodate up to 4 budgets.
When will applicants be notified if opt-out strategies and activities are approved?
Applicants will be notified of approved activities in the Notice of Award (NOA).
Is there an email address we can use to submit questions that come up during the writing of the grant?
While CDC cannot provide assistance in the development of grant applications, general questions should be directed to DVH_FOA@cdc.gov. All questions and responses will be available and posted on DVH’s website.
Can the templates use 10 point font, or does the entire application need to be 12 point font (as instructed in the NOFO)?
As stated in the NOFO on page 74, the application should be written in 12 point font. The templates have been redesigned using 12 font.
Other than CVs, MOUs, etc., can other documents be included as Appendices?
Yes. Additional documents can be added as appendices if needed to enhance understanding of your program.
Given that page limits were expanded from 20 to 30 pages after the October 14th informational webinar, how will CDC ensure fairness in the review process for those who had already submitted applications in accordance with previous 20-page limit?
CDC recognizes the challenge of obtaining approval for application packages before submitting, including timelines that vary by jurisdiction. Applicants may submit an unlimited number of revisions to their applications via Grants.gov prior to the application deadline (December 1, 2020, 11:59 PM ET). OGS will accept the most current application submitted. Page count is not a factor in the scoring of applications.
Can funds awarded through PS21-2103 be used to provide an honorarium for presenters/trainers for viral hepatitis trainings with providers?
Yes. Funding under PS21-2103 can be used to support honoraria for provider trainings about viral hepatitis.
During the webinar, it was stated that incentives can be provided under the new NOFO. Can remaining PS1702/03 funds also be used for incentives if the same guidelines are followed?
Remaining PS17-1702 and PS17-1703 funds may be used for activities specified under PS17-1702 and PS17-1703, respectively. Questions regarding incentives under PS17-1702/03 should be directed to the Project Officer for that respective jurisdiction.
Are local health departments eligible to apply for this funding as bona fide agents of the County? If both a local jurisdiction and the state in which that jurisdiction is located apply for PS21-2103, will the funding for the local and/or the state applicant be reduced? Will one or both applicants be less likely to be funded?
Applications from local and state jurisdictions will be evaluated independently using the same criteria. Applicants from local jurisdictions should clearly explain how each activity is coordinated with the state, and vice versa. Page 27 of the NOFO states that: “Local health department applicants are required to submit a letter of agreement/MOU between the appropriate state and local health department delegating authority for surveillance to the local health department and detailing how surveillance data will be reported to CDC.”
Can NOFO funds be used to supplement the salary of a clinician who would be tasked with mentoring and providing consultative services to external clinicians who are managing patients with HCV infection (as has been done with similar funded activities in TB and HIV)?
Page 54 of the NOFO states, “Recipients may not use funds for clinical care except as allowed by law.” This includes clinical consultation for individual clinicians caring for a specific patient. However, if funds are used to support case-based learning such as ECHO or similar models that include mentoring and consultation for all clinician attendees of the ECHO session, then the expense would be acceptable.
The Evaluation and Performance Management section of the NOFO (pages 59 and 60) states that a maximum of 35 points can be assigned, yet each of the three components is assigned 35 points. Should this be addressed specifically for each component (for a maximum of 35 points per section) or under one heading in the application that is worth a maximum of 35 points?
Each component will be scored separately, therefore, evaluation for that component should be included in that section. See page 39 for further information on the workplan (includes evaluation). See https://www.cdc.gov/hepatitis/policy/FO-CDC-RFA-PS21-2103.htm for workplan templates (includes evaluation). Please refer to page 74 of the NOFO for additional information on page limits. In addition, make sure that sections are clearly labelled. Workplan sections should be clearly labelled (NOFO, page 39) and the Project Narrative must include all of the following headings (including subheadings): Background, Approach, Applicant Evaluation and Performance Measurement Plan, Organizational Capacity of Applicants to Implement the Approach, and Work Plan (NOFO, page 49).
Can applicants budget for travel to meetings and/or conferences?
Applicants may budget for travel to relevant meetings that are currently planned as in-person. Contingency plans should be noted in the budget justification for travel to in-person meetings that may be cancelled or converted to virtual only later due to COVID-19 or other circumstances. Budget will not be approved for travel to meetings that have announced cancellation or plans to meet virtually. Funded recipients must provide a revised budget within a timeframe specified in the Notice of Award.
How does CDC envision that health departments can increase testing within federally qualified health centers (FQHCs) and other agencies given that funding for these settings is unavailable and the clinics themselves are financially burdened?
Health departments should consider discussing state and federal policies for testing and treatment for viral hepatitis in their viral hepatitis elimination task force or committee as part of planning for viral hepatitis elimination. Existing federal policies require that insurance cover vaccines and testing for viral hepatitis without charging a deductible or co-pay (see https://www.hhs.gov/hepatitis/policies-and-guidelines/affordable-care-act/index.html). During development of a state viral hepatitis elimination plan, states can evaluate what steps are most realistic and feasible to promote testing for viral hepatitis in the jurisdiction. CDC will offer technical assistance and facilitate peer-to-peer support for states working on hepatitis elimination plans.
Given that vaccines are strictly handled by jurisdictions’ immunization programs rather than the viral hepatitis coordinator, how does CDC envision coordinating the NOFO’s vaccine-related activities within a jurisdiction, including vaccine distribution, in settings that have been uncharted?
CDC’s Immunization Services Division funds highly effective public health programs, including those focused on perinatal hepatitis B prevention and universal childhood vaccinations such as hepatitis A and hepatitis B vaccines. These programs have been critical for reducing new cases of hepatitis A and B in the United States in children and young adults and promote eventual elimination of hepatitis B. Some jurisdictional immunization programs have helped provide vaccine to control outbreaks of hepatitis A among persons who use drugs and persons experiencing homelessness.
As part of this funding opportunity, grantees will develop a viral hepatitis elimination plan in collaboration with appropriate stakeholders from the jurisdiction. The jurisdictional immunization program will necessarily play a critical role in the jurisdictional viral hepatitis elimination plan; however, immunizations can also be funded by health insurance (see https://www.hhs.gov/hepatitis/policies-and-guidelines/affordable-care-act/index.html). The jurisdiction should discuss the best path forward for the jurisdiction and document their plans in the state viral hepatitis elimination plan. CDC will offer technical assistance and facilitate peer-to-peer assistance to support states in development of jurisdictional viral hepatitis elimination plans.
Page 49 of the NOFO states that the project narrative “must include all of the following sections,” which includes an Applicant Evaluation and Performance Measurement Plan; a Workplan is also mentioned later in the NOFO. Does the template provided on Page 39 (“Sample-Work-Evaluation-Plan Template”) combine the two required sections?
Applicants may use any strategy that clearly communicates how they plan to evaluate their proposals. The template included in the NOFO provides one option (page 39). Additional templates designed to address the workplan and evaluation components are found at: https://www.cdc.gov/hepatitis/policy/FO-CDC-RFA-PS21-2103.htm. Applicants should make sure that sections are clearly labelled, including those within the workplan. The Project Narrative must include all the following headings (including subheadings): Background, Approach, Applicant Evaluation and Performance Measurement Plan, Organizational Capacity of Applicants to Implement the Approach, and Work Plan.
The CDC workplan template is different from the one provided on page 39 of the NOFO, where applicants are asked to provide a SMART objective for each activity. That column is missing from the template in the FAQ section. Does that mean the SMART objective section is optional?
Page 39 of the NOFO states: “An example of a workplan template follows; the jurisdiction may use any template that clearly links program activities to expected outcomes.” This means that the jurisdiction can use any workplan template that clearly links program activities to expected outcomes. If desired, SMART Objectives may be included in the “Process Measures” column of the template.
If a program outside of viral hepatitis is responsible for hepatitis A surveillance, prevention, and outbreak response, can a health department exclude these responsibilities from the proposed activities associated with this NOFO? Is providing justification that another program is specifically funded to do the prescribed activities enough? If so, can you provide more detail about how states should communicate the desire to exclude hepatitis A surveillance and associated outbreak response activities that are and will be performed by staff in their general communicable diseases program?
Page 26 of the NOFO states, “In order to achieve the goals of the project, recipients are required to collaboratively partner with CDC and other CDC-funded state and local health department surveillance and prevention programs such as, but not limited to, HIV, STD, immunization, public health emergency preparedness and response, health care associated infections, comprehensive cancer prevention programs, and injury prevention.” PS21-2103 applicants may describe existing activities and funding that address hepatitis A surveillance and outbreak response, noting whether and how existing programs or activities proposed under PS21-2103 will address remaining hepatitis A surveillance and outbreak response activities, including but not limited to coordination on strategies to improve completeness of case reporting, response to hepatitis A outbreaks driven by person-to-person transmission, and investigation of cases involving co-infection with hepatitis A, B and C. Further, page 11 of the NOFO states, “Recipients can request to opt out of selected required activities by providing a strong justification, which must be based on program need, resources and/or policies. Approval will be made after review of the application.”
Component 1: Core Viral Hepatitis Surveillance
With the amount of resources* available for Component 1, how should jurisdictions prioritize activities?
The focus in years 1–3 should be on short-term outcomes, to include
- establishing jurisdictional framework(s) for outbreak detection and response;
- improving current monitoring of the burden and trends in hepatitis A, acute hepatitis B, and acute and chronic hepatitis C, to include improving completeness of case reports and reporting; and
- increasing public health reporting of chronic and perinatal hepatitis C virus (HCV) and chronic hepatitis B virus (HBV) infections and undetectable HCV-RNA and HBV-DNA laboratory results.
*Note that Strategy/Activity 1.3 is dependent on availability of funding. Recognizing that surveillance activities are resource intensive, CDC identified activities that could be delayed until supportive funding was available.
What if a disease is not reportable in my state?
One of the short-term outcomes for Component 1 is to increase public health reporting of perinatal hepatitis C. However, you will not be responsible for notifying CDC of hepatitides that are not reportable in your state. If you receive reports of any hepatitides that are not reportable in your state, please notify CDC of those reports. Your application should indicate which hepatitides are currently reportable in your jurisdiction, the hepatitides that are being considered to place on the list of reportable diseases, and whether your health department receives negative laboratory results for HBV and/or HCV.
For metric 1.2.3.a (90% of cases reported within 90 days of case investigation date), what if cases are not investigated at all? Should they be included in the denominator?
The goal is for 90% of cases to be reported within 90 days of the jurisdiction receiving the first report (e.g., a positive laboratory result received via electronic laboratory reporting). Cases may be classified initially based on available information (e.g., by an automated algorithm) and reported within 90 days on this basis. If further investigated later, the case classification may be revised in a subsequent notification to CDC. Consultation with the Surveillance Officer assigned to the jurisdiction may provide further guidance based on the jurisdiction’s specific case reporting laws and regulations.
Is it permissible to offer incentives to clients participating in Components 3.1, 2.2, and 2.3?
Yes. It is permissible for the recipient to offer non-cash incentives to clients participating in PS21-2103 Component 2.2, 2.3, and 3.1 activities. Recipients will be expected to track the incentives given. There should be a log or record showing the cost of gift cards, notating who the gift cards were issued to, the date, and the signature of the persons receiving the incentive. Gift cards should be vendor specific, limiting the use for general purpose purchases (avoiding AMEX/VISA/Master Card gift cards). All gift cards amounts should be targeted at $5 and $10 and should not exceed a $25 cap.
What is the timeline for the full implementation of the Hepatitis Message Mapping Guide?
The Hepatitis Message Mapping Guide is available for implementation. Jurisdictions progress through phases of implementation (pilot, receiving technical assistance, onboarding, and production). The September 2020 issue of NMI Notes indicates that six states are receiving technical assistance for hepatitis, three are onboarding, and 22 are in production. The NNDSS Modernization Initiative FAQ provides the following information about the Message Mapping Guide timeline for implementation, under the section “Retirement of the Legacy NNDSS Portfolio” (excerpt below from (https://www.cdc.gov/nmi/faq.html#Retirement):
How does CDC advise measuring the hepatitis B care cascade when treatment is not recommended for everyone?
Guidance for measuring the hepatitis B care cascade will be developed in collaboration with jurisdictions and national partner organizations.
Strategy 1.2 addresses acute hepatitis B and acute and chronic hepatitis C, but one of the short-term outcomes speaks to perinatal hepatitis C and chronic hepatitis B. At the same time, strategy 1.3 addresses chronic hepatitis B and perinatal hepatitis C. Under which strategy should applicants detail their plans for chronic hepatitis B and perinatal hepatitis C?
The Strategy 1.2 short term outcome to increase public health reporting of chronic hepatitis B and perinatal hepatitis C supports activities to increase reporting from providers to the jurisdiction and from the jurisdiction to CDC. Strategy 1.3 addresses monitoring (i.e., use of the surveillance data for chronic hepatitis B and perinatal hepatitis C to describe case distribution and trends geographically, demographically, and by risk factors to inform public health action). Applicants should detail activities for chronic hepatitis B and perinatal hepatitis C surveillance under the relevant strategy.
Page 27 of the NOFO states “Local health department applicants are required to submit a letter of agreement/MOU between the appropriate state and local health department delegating authority for surveillance to the local health department and detailing how surveillance data will be reported to CDC.” Do local health departments (LHDs) need to submit surveillance data directly to CDC or just describe how surveillance data flows from local to state to CDC?
The application should describe how the LHD and state health department (SHD) will collaborate and how data will be submitted to CDC within the laws and regulations of the state and local jurisdictions and under authority of the LHD and SHD for notifying CDC of viral hepatitis cases.
Component 2: Core Viral Hepatitis Prevention Activities
Who should I partner with for Component 2?
Recipients are required to establish, build, and/or maintain other collaborative relationships consistent with the goals of the project. These collaborations include (but are not limited to)
- jurisdictional public health agency leadership;
- state Medicaid and mental health agencies;
- state, regional and/or local correctional agencies;
- state/local harm reduction coordinators and harm reduction coalitions;
- professional associations representing provider and health care organizations;
- tribal communities and tribal health providers;
- health advocates (such as community-based organizations and persons with lived experience);
- academic medical centers, laboratories, provider champions, and health systems serving target populations;
- state and local health departments; and
- state and local public health associations.
How flexible are the funds for Component 2 in this NOFO
Funds can be used to purchase hepatitis testing kits and laboratory equipment. Funds can be used to support syringe services programs (SSPs) if a Determination of Need (DON) is in place for the jurisdiction. CDC federal funds can be used to support certain components of SSPs, including:
- staffing;
- supplies (e.g., alcohol pads, sterile water, and cotton);
- testing kits for viral hepatitis and HIV;
- syringe disposal services;
- navigation services to ensure linkage to services;
- communication, outreach, and educational materials;
- condoms;
- planning and evaluation activities, to include a needs assessment and/or special studies to identify
- resource needs to facilitate service delivery consistent with this NOFO,
- staff training and development needs,
- client needs, such as hours of service availability, types of services needed by clients, client perception of services offered, and perception of stigma and compassion during service delivery), and
- other assessment needs to design services consistent with this NOFO;
- evaluation studies to document impact of grant-funded interventions.
The CDC Program Guidance for Implementing Certain Components of Syringe Services Programs, 2016, provides specific procedures for CDC-funded grantees. Further information on syringe services programs is available.
How will CDC assist with developing relationships with partners such as community health centers (CHCs)?
CDC is currently working with various partners to facilitate and enhance collaboration between health departments and CHCs.
Component 2.1, Outcome 2.1.3. refers to increased HCV and/or HBV testing in health care systems. Is there a specific definition that applicants should use for what qualifies as a health care system?
The PS21-2103 NOFO, page 81, provides a list of definitions. The definition of health systems is “organizations of people, institutions, and resources that deliver health care services to meet the health needs of target populations.” The specific definition of a health care system evolves over time; example definitions are available on the Agency for Health care and Research Quality (AHRQ) website.
Can funds from Component 2 be used for provider trainings to design and offer CME courses where a state and a city within the state apply for funding, but surveillance is mostly handled by the state; can the city applicant defer some of the Component 1 outcomes to the state applicant?
Yes, funds can be used for provider trainings and CME courses and the applicant needs to explain it clearly in the budget narrative/justification. Local health department applicants are required to submit a letter of agreement/MOU between the appropriate state and local health department delegating authority and provide details on the activities that will be conducted.
For component 2, do the high-impact settings need to be identified within the application, or are jurisdictions expected to identify those settings and partners during Year 1?
For component 2.2, which is required but contingent on funding, applicants must focus activities in one or more high-impact settings working in collaboration with relevant organizations to provide viral hepatitis testing and referral to care. If a jurisdiction has already identified partner(s) and high-impact settings, this information should be included in the application; if not, jurisdictions should describe how the settings and partners will be identified in the future.
Can you provide clarification on whether specific NOFO components (e.g., Components 2.2-2.3 and Component 3) will be funded sequentially?
Components 2.2, 2.3, and 3 are required elements in the application but are contingent on availability of funds. At this time, it is unknown whether funding will be available for activities currently listed as “contingent on funding;” therefore, individual components will not be funded in a linear or sequential manner.
For Component 2.1 (Provider Training Strategy), can funds be allocated for CME courses?
Yes, funds can be used for provider trainings and CME courses if this activity is clearly identified in the application and in the budget narrative/justification.
For Components 2.2-2.3, can jurisdictions conduct activities in more than one high-impact setting?
Yes, on Page 18 of the NOFO it states that applicants can focus activities in one or more high-impact settings.
Component 2 implies that jurisdictions are required to address both hepatitis B and C, but it also specifically states that decisions to exclude certain components must be justified. Can this be clarified?
Applicants are encouraged to address both hepatitis B and C. However, as stated on Page 11 of the NOFO, applicants can request to opt out of selected required activities by providing a strong justification, which may be based on program need, resources, and/or policies.
For Component 2.3.b., can funding be used to support the purchase of hepatitis vaccine?
No. Page 55 of the NOFO clearly states that funds cannot be used to purchase drugs and/or vaccine. Note that federal policy requires coverage of vaccination without deductible or co-pay; consider enrolling clients in insurance coverage to fund vaccine: https://www.hhs.gov/hepatitis/policies-and-guidelines/affordable-care-act/index.html
Must jurisdictions be granted approval through the SSP determination-of-need process before using funds to support the activities associated with Component 2.3.b?
For Component 2.3.b, which outlines establishment of vaccine delivery teams in high impact settings, a determination of need (DON) is required before activities are undertaken, because vaccine teams are considered federal support to the comprehensive services that an SSP provides. The DON is applicable to SSP’s only and not for other high impact settings.
Component 3: Special Projects: Prevention, diagnosis, and treatment related to the infectious disease consequences of drug use
What is the purpose of Component 3 funding?
The purpose of Component 3 funding is to enable jurisdictions to improve access to services for persons who inject drugs (PWID) in settings disproportionately affected by drug use. Specifically, the funding required delivery of a “PWID service bundle” in settings serving PWID. The PWID service bundle is defined as evidence-based services, including
- sufficient sterile injection equipment to cover all injections;
- disposal of used injection paraphernalia;
- assessment for opioid use disorder (OUD) and linkage to medication assisted treatment (MAT);
- naloxone provision and training;
- testing for HCV, HBV, and HIV;
- vaccination for hepatitis A and hepatitis B;
- treatment for infectious diseases (viral, bacterial and fungal); and
- HIV pre-exposure prophylaxis (PrEP).
Settings serving PWID include
- syringe services programs (SSPs),
- substance use disorder (SUD) treatment centers,
- correctional facilities,
- emergency departments,
- hospitals, and
- any venue with a demonstrably high prevalence of PWID clients.
Who is eligible for Component 3 funding?
Like Components 1 and 2, this funding opportunity limits competition to state, county, and city or township governments and their bona fide agents. These entities have the statutory authority to conduct viral hepatitis surveillance activities and to design, implement, and evaluate prevention programs and policies that impact communities.
Can my jurisdiction apply for Component 3 without applying for Components 1 and 2?
No. Applicants must apply for Components 1 and 2 to be considered for Component 3.
Who should I partner with for Component 3?
At the jurisdiction level, many of the same partners for Component 2 will also be valuable partners for Component 3, especially state Medicaid, the state Harm Reduction Coalition, the state mental health or substance use disorder treatment agency, payors, and public health programs for immunization, HIV/AIDS, and injury. If Component 3 is implemented in a community, evidence of strong partnerships at the community level will be important. Community-level partners include
- SSPs,
- SUD treatment programs,
- hospitals,
- correctional facilities,
- Federally Qualified Health Centers and Rural Health Centers, and
- other community partners serving PWID.
What care models can be funded?
Integrated care models are most effective for service delivery for PWID. Information on integrated care models is available from the Substance Abuse and Mental Health Services Administration. Patient navigators can also be funded to link PWID to needed services. Patient navigators are employees who facilitate access from one community-based organization to another, help clients address barriers to care, and track referral outcomes. Patient navigators may be peers, social workers, outreach workers, or anyone who is familiar with the local landscape of community-based organizations and capable of helping clients access needed care.
A peer navigator is a type of patient navigator who also serves as a role model. Peer navigators have similar life experience and a shared community membership with the populations with whom they work. Peers are trained, often paid, professional staff members rather than volunteers. Their work includes
- case finding and community outreach,
- routine appointment reminder phone calls,
- accompaniment to appointments,
- transportation assistance,
- referrals and associated follow-up, and
- adherence education and support.*
*Adapted from: AIDS United. Best Practices for Integrating Peer Navigators into HIV Models of Care. Washington, DC. 2015. https://www.aidsunited.org/data/files/Site_18/PeerNav_v8.pdf [ PDF – 26 pages]
Does the service bundle for Component 3 have required elements? Do we have to include all items on the NOFO list? There is a challenge with syringe services programs (SSPs) because of laws regarding them in our state.
Yes. The PWID service bundle does have required elements. The PWID service bundle should include access (directly or through referral) for all the elements. For more detailed information, applicants may refer to Page 24 of the NOFO.
Our determination of need (DON) was approved in January 2020. Do we have to submit a request each year or will that response be sufficient for the PS21-2103 NOFO?
Once a jurisdiction receives CDC approval of the DON, there is no need for reapproval. However, a jurisdiction that does not have a DON in place can submit/resubmit an application with new supporting evidence justifying the need.
Is the average year 1 award for Component 3 $300,000 as documented on the website and in the FAQs posted on the website, or $275,000 as documented in the published NOFO?
CDC-RFA-PS21-2103 Component 3 funding is being updated in the published NOFO to reflect the correct average year 1 award amount of $300,000.
Will Component 3 be competitively awarded each year? If so, is if there any way to make it competitively awarded for three years of funding, for instance?
Yes, each yearly application will be competitively reviewed, ranked, and scored; however, to ensure continued activities in years 1-3, CDC reserves the right to fund out of rank and order based on criteria outlined in Component 3 of the NOFO.
Can funds for Component 3 be used to pay for clinic staff time? Specifically, can the funds be used to pay for a nurse’s time to conduct a blood draw and the admin fee for the blood draw?
Under “Funding Restrictions” on page 54, the NOFO states, “Recipients may not use funds for clinical care except as allowed by law.”
When applying for Component 3, should jurisdictions integrate the sections (e.g., Outcomes and Strategies) with Components 1 and 2, or should each section be listed separately under each Component?
While strategies in Components 1, 2, and 3 should be coordinated, each component of a funding request will be scored independently. Applicants are allowed up to 10 pages for each component to describe specific project narrative subsections (Approach, Evaluation and Performance Plan, and Work Plan); therefore, outcomes and strategies for each component should be addressed separately. See page 74 of the NOFO for additional information on page limits. See page 39 for additional information on the workplan. See also workplan templates available at https://www.cdc.gov/hepatitis/policy/FO-CDC-RFA-PS21-2103.htm.