National Progress Report 2025 Goal: Reduce estimated* new hepatitis B virus infections by ≥20%
Estimated* new hepatitis B virus infections
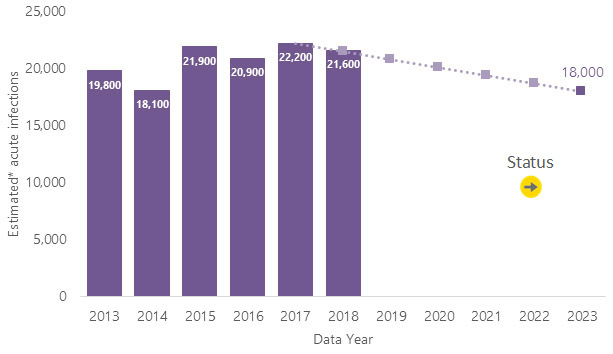
Source: CDC, National Notifiable Diseases Surveillance System
*The number of estimated viral hepatitis infections was determined by multiplying the number of reported cases by a factor that adjusted for under-ascertainment and under-reporting (1–2).
Summary of Findings
The estimated number of new hepatitis B virus (HBV) infections has been relatively stable since 2015. In 2018, there were 21,600 estimated new infections, slightly above the 2018 target of 21,500 estimated infections.
Reduction needed to meet 2025 goal: A 16.7% reduction from the estimated number of new HBV infections in 2018 is needed to meet the 2025 goal of 18,000 estimated infections.
This reduction can best be achieved by
- Promoting implementation of pediatric and adult vaccine recommendations through provider education, strategic partnerships, and other measures.
- Building capacity for states to collect and use a core set of surveillance data to detect at-risk populations and gaps in vaccination coverage.
- Conducting prevention research to demonstrate how best to provide hepatitis B vaccination, testing, and treatment as part of a comprehensive set of interventions for persons who inject drugs.
- Encouraging unvaccinated persons to use HHS’s Adult Vaccine Finder to locate providers of recommended adult vaccines and get immunized against hepatitis B.
Technical Notes
Data Sources: CDC, National Notifiable Diseases Surveillance System (NNDSS)
Numerator: Number of estimated new (acute) HBV infections
Denominator: Not applicable
Indicator Notes: (1) The NNDSS is a nationwide collaboration that enables all levels of public health to share notifiable-disease-related health information. Surveillance for viral hepatitis through NNDSS is based on case definitions developed and approved by the Council of State and Territorial Epidemiologists (CSTE) and CDC. Reported cases of acute viral hepatitis B are required to meet specific clinical and laboratory criteria. Estimated infections are based on laboratory-confirmed cases of acute viral hepatitis. Acute hepatitis B is reportable in all jurisdictions. Health-care providers, hospitals, and/or laboratories report cases to the local or state health department, and states voluntarily submit reports or notify CDC of newly diagnosed cases of hepatitis B that meet the surveillance case definition. To account for under-ascertainment and under-reporting, the number of reported cases is multiplied by 6.5. The methods for developing the multiplication factor are documented in Klevens, et al (2) with corrected multipliers developed by CDC (1).
Goal Setting: The 2025 goal of 18,000 estimated cases is consistent with CDC’s Division of Viral Hepatitis 2025 Strategic Plan. Annual targets assume a constant (linear) rate of change from the observed baseline (2017 data year) to the 2025 goal (2023 data year).
Limitations: The number of estimated infections is based on a simple, probabilistic model for estimating the proportion of patients who were symptomatic, received testing, and were reported to health officials in each year (2). This constant multiplier may not account for variations over time in under-reporting and under-ascertainment due to public and provider awareness, changes in laboratory and diagnostic techniques, and changes in the definition of the condition.
References
- Centers for Disease Control and Prevention. Viral Hepatitis Surveillance—United States, 2018. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2020. Available at: https://www.cdc.gov/hepatitis/statistics/2018surveillance/pdfs/2018HepSurveillanceRpt.pdf.
- Klevens RM, Liu S, Roberts H, Jiles RB, Holmberg SD. Estimating acute viral hepatitis infections from nationally reported cases. Am J Public Health. 2014;104(3):482-7.