Safe and effective vaccine that prevents cancer continues to be underutilized
Latest vaccination coverage estimates for adolescents show only small increase for HPV vaccine
Press Release
Embargoed Until: Thursday, July 24, 2014, 1:00 PM, ET
Contact: Media Relations
(404) 639-3286
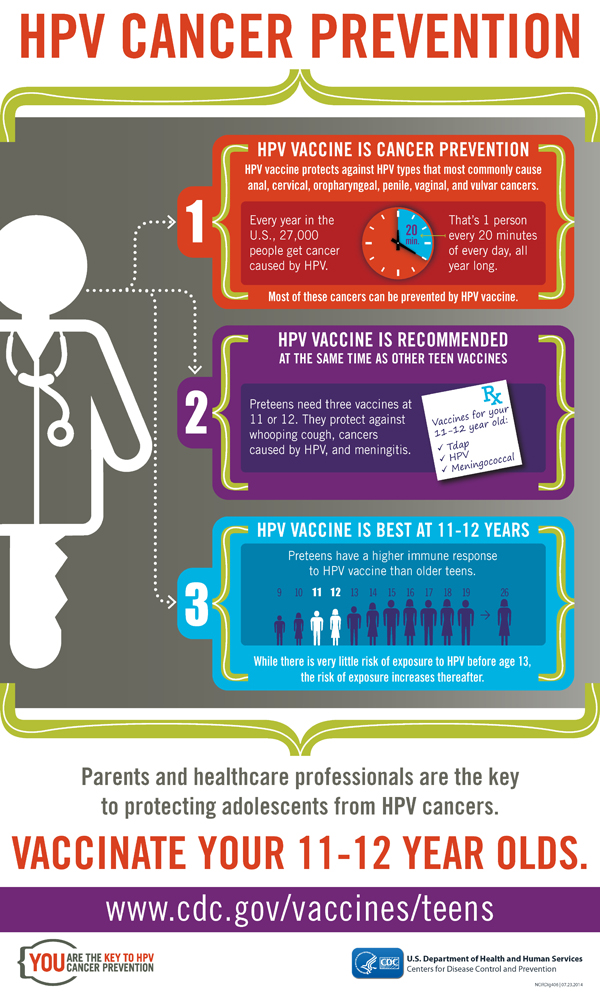 [PDF]
[PDF]HPV Cancer Prevention. Entire Infographic
CDC officials announced today that the number of girls and boys aged 13-17 years receiving human papillomavirus (HPV) vaccine remains unacceptably low despite a slight increase in vaccination coverage since 2012, according to data from CDC′s 2013 National Immunization Survey-Teen (NIS-Teen) published in this week′s Morbidity and Mortality Weekly Report (MMWR).
HPV vaccine prevents various forms of cancer, but HPV vaccine remains underutilized. There is a substantial gap between the number of adolescents receiving tetanus, diphtheria, and pertussis (Tdap) vaccine and the number receiving HPV vaccine. It is estimated that only 57 percent of adolescent girls and 35 percent of adolescent boys received one or more doses of HPV vaccine. However, nearly 86 percent of adolescents had received one dose of Tdap vaccine.
These gaps in coverage indicate missed opportunities to vaccinate boys and girls with HPV vaccine at the same time as other routinely recommended adolescent vaccines like Tdap and meningococcal vaccines. “The high coverage rate of Tdap vaccine shows us that it is certainly possible to reach our goal of vaccinating 80 percent of adolescents against cancers caused by HPV,” said Dr. Anne Schuchat, assistant surgeon general and director of CDC’s National Center for Immunization and Respiratory Diseases.
CDC estimates that if missed opportunities to vaccinate adolescent girls before their thirteenth birthdays were eliminated, 91 percent of adolescent girls would have some protection from cancers caused by HPV infection. “Pediatricians and family physicians are uniquely situated to prevent missed opportunities by giving HPV vaccine during the same visit they give Tdap and meningococcal vaccines,” said Schuchat.
Only one-third of adolescent girls received the recommended series of three doses of HPV vaccine. “It’s frustrating to report almost the same HPV vaccination coverage levels among girls for another year,” said Schuchat. “Preteens need HPV vaccine today to be protected from HPV cancers tomorrow.”
The data also illustrate the influential role a clinician’s recommendation plays in whether or not parents choose to vaccinate their children. For parents that had their daughters vaccinated against HPV, 74 percent received a recommendation from a health care professional, compared to 52 percent of parents who did not have their daughters vaccinated. For boys, the difference is even more staggering as 72 percent of parents who chose to vaccinate their sons received a recommendation, compared to 26 percent of parents who did not have their sons vaccinated. Not receiving a clinician’s recommendation for HPV vaccine was one of the five main reasons parents listed during the NIS-Teen interviews for not vaccinating their children against HPV.
Parents also reported safety concerns as a reason for not having their children vaccinated against HPV. In the eight years of post-licensure vaccine safety monitoring and evaluation conducted independently by federal agencies and vaccine manufacturers, and after 67 million doses of HPV vaccine have been distributed, no serious safety concerns have been linked to HPV vaccination. According to today’s MMWR article, the most commonly reported symptoms after receiving an HPV vaccine include injection-site reactions such as pain, redness, and swelling; other commonly reported symptoms include dizziness, fainting, nausea, and headache. No new safety concerns have arisen since 2009 when the summary of the first 2.5 years of post-licensure reporting to the Vaccine Adverse Events Reporting System (VAERS) was published.
CDC urges health care professionals to give a strong recommendation for all of the adolescent vaccines recommended for boys and girls aged 11 or 12 years. Clinicians should recommend HPV vaccine as they would recommend Tdap and meningococcal vaccines. Reviewing vaccination status at every health care visit is another strategy doctors and nurses should take to increase vaccine coverage.
All preteens need one dose of Tdap vaccine, one dose of meningococcal vaccine, and three doses of HPV vaccine to be fully protected against serious diseases, including HPV cancers. A second dose of meningococcal vaccine is needed at age 16. Parents and caregivers are encouraged to ask about vaccination every time they take children for a health care visit. If a preteen boy or girl (aged 11 or 12 years) has not received HPV, Tdap, and meningococcal vaccines, make an appointment to get him or her vaccinated.
The NIS-Teen is a random-digit-dialed survey of parents and guardians of teens 13–17 years old and in 2013, included data for more than 18,000 adolescents. The telephone survey is followed by verification of records with health care professionals.
The Affordable Care Act was created to expand access to coverage, lower health care costs, and improve health care quality and coordination. Through the Affordable Care Act, more Americans can get access to the health care coverage that fits their needs and budget, including important preventive services such as the HPV vaccine, tobacco use screenings and tobacco cessation, blood pressure screening and aspirin therapy, cancer screenings, and other services that may be covered with no additional costs for new health plans and in states that are newly expanding their Medicaid programs. Visit Healthcare.gov or call 1-800-318-2596 (TTY/TDD 1-855-889-4325) to learn more.
Today’s article will be available on the Morbidity and Mortality Weekly Report website at http://www.cdc.gov/mmwr/ after the embargo lifts at 1 p.m. ET.
For more information on the National Immunization Survey (NIS), please visit:
http://www.cdc.gov/nchs/nis.htm
Preteen and Teen Vaccines:
http://www.cdc.gov/vaccines/teens
HPV Vaccine Resources for Healthcare Professionals:
http://www.cdc.gov/vaccines/YouAreTheKey
