CDC’s Sickle Cell Data Collection (SCDC) Data Brief: Hydroxyurea
Hydroxyurea Use Among Medicaid Beneficiaries with Sickle Cell Disease in California and Georgia, 2006–2016
During 2006–2016, the use of hydroxyurea (HU) among Medicaid beneficiaries with sickle cell disease (SCD) who lived in California or Georgia increased. However, many beneficiaries with severe complications of SCD do not use HU.
HU was approved by the FDA in 1998 for the treatment of severe SCD in adults and in 2017 for the treatment of pediatric patients. It can decrease several complications of SCD, including severe acute pain episodes and acute chest syndrome, and may lead to fewer hospitalizations and improved survival.
How many Medicaid beneficiaries with SCD in California and Georgia are taking HU?
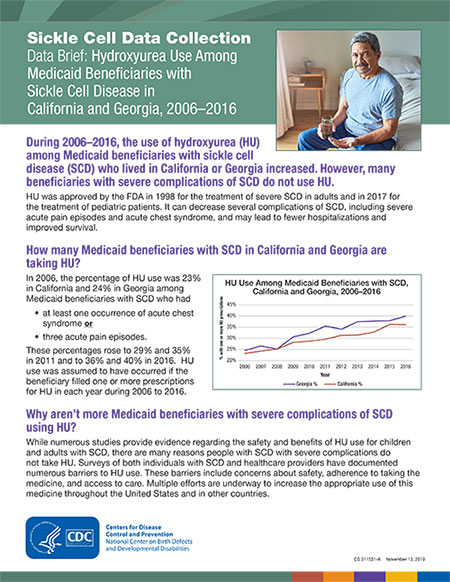
In 2006, the percentage of HU use was 23% in California and 24% in Georgia among Medicaid beneficiaries with SCD who had
- at least one occurrence of acute chest syndrome or
- three acute pain episodes.
These percentages rose to 29% and 35% in 2011 and to 36% and 40% in 2016. HU use was assumed to have occurred if the beneficiary filled one or more prescriptions for HU in each year during 2006 to 2016.
Why aren’t more Medicaid beneficiaries with severe complications of SCD using HU?
While numerous studies provide evidence regarding the safety and benefits of HU use for children and adults with SCD, there are many reasons people with SCD with severe complications do not take HU. Surveys of both individuals with SCD and healthcare providers have documented numerous barriers to HU use. These barriers include concerns about safety, adherence to taking the medicine, and access to care. Multiple efforts are underway to increase the appropriate use of this medicine throughout the United States and in other countries.
Sickle Cell Data Collection (SCDC)
The Sickle Cell Data Collection (SCDC) program collects health information about people with sickle cell disease (SCD) in the United States in an effort to
- study the long-term trends in diagnosis, treatment, and healthcare access for people with SCD, and
- inform policy and healthcare standards to improve and extend the lives of people with SCD.
At the time this data brief was produced, SCDC was funded through the support of the CDC Foundation, Pfizer Inc., Global Blood Therapeutics, Sanofi, and Doris Duke Charitable Foundation.
For More Information:
- Sickle Cell Disease: https://www.cdc.gov/ncbddd/sicklecell
- Sickle Cell Data Collection: https://www.cdc.gov/ncbddd/hemoglobinopathies/scdc.html
- FDA Prescribing Information for Hydroxyurea (pediatric patients): https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208843s000lbl.pdf [352 KB / 25 pages]
- FDA Prescribing Information for Hydroxyurea (adult patients): https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/016295s041s042lbl.pdf [470 KB / 33 pages]
- NHLBI’s Evidence-Based Management of Sickle Cell Disease: Expert Panel Report, 2014: https://www.nhlbi.nih.gov/health-topics/evidence-based-management-sickle-cell-disease