Blood Lead Safety Alert
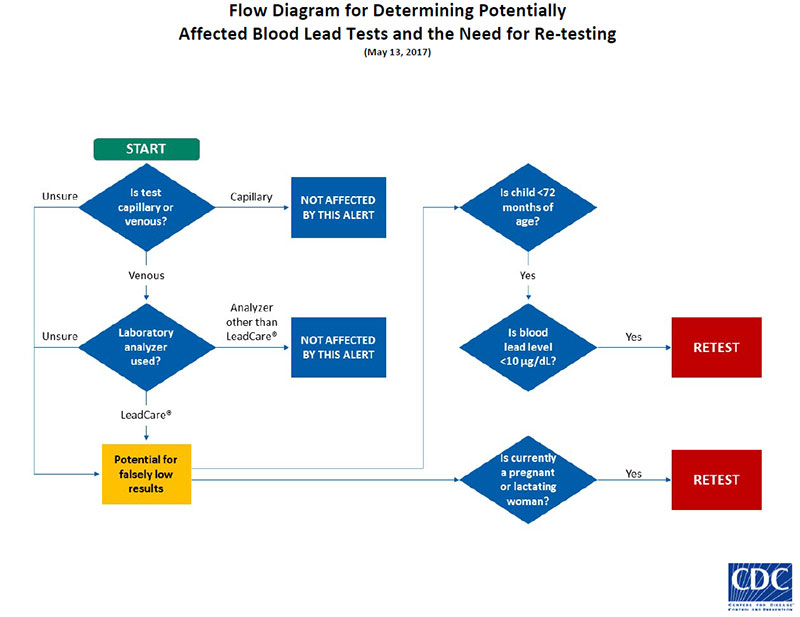
The U.S. Food and Drug Administration (FDA) has issued a safety alert about the use of Magellan Diagnostics’ LeadCare® analyzers with venous blood samples because they might result in false negative test findings. This safety alert does not apply to capillary blood lead test results collected by fingerstick or heelstick. At this time, we don’t know how many blood lead tests related to this safety alert may be affected.
CDC is working with FDA and public health officials throughout the United States to determine where the analyzers were used and which blood lead test results might be affected. At this time, we don’t know how many blood lead test results might be affected.
CDC recommends health care providers re-test children who
1) are younger than 6 years (72 months) of age at the time of the safety alert (May 15, 2017), and
2) had a venous blood lead test result of less than 10 micrograms per deciliter (µg/dL) from a Magellan Diagnostics LeadCare® analyzer at an onsite or offsite laboratory.
If the provider is certain that analyzers other than those affected by the safety alert were used to analyze venous blood samples, a re-test is not recommended.
If re-testing indicates blood lead levels in excess of the CDC reference value (www.cdc.gov/nceh/lead/prevention/blood-lead-levels.htm) or appropriate state or local action level, the health care provider or public health official should refer to CDC guidelines or state/local guidelines for appropriate follow up action (www.cdc.gov/nceh/lead/advisory/acclpp/actions-blls.htm).
CDC also recommends that health care providers re-test pregnant or lactating women who had a venous blood sample.
We understand that parents of children and other people affected by this safety alert might be concerned about what this means for their child’s health or their health. CDC recommends parents and others who are affected discuss with their health care provider or health department whether re-testing is needed.
Laboratory tests analyzed by inductively coupled plasma-mass spectrometry (ICP-MS) or graphite furnace atomic spectrometry (GFAAS) (also known as electrothermal atomic absorption spectrometry [ETAAS]) are not expected to result in false-negatives.