July 2022

CDC releases short version of the mpox case report form
On July 5, 2022, CDC announced a short version of the mpox case report form to state, local, and territorial public health jurisdictions to reduce the burden of submitting case data.
CDC understands the challenges jurisdictions face in rapidly providing CDC with complete, final information for every data element in the case report form, particularly at the beginning of an outbreak response. The short case report form will make mpox case reporting and notification easier and more efficient for jurisdictions.
CDC is reducing the amount of initial information we are asking jurisdictions to provide for each new case. Jurisdictions will have the opportunity to update their previously submitted case data as their case investigations continue.
CDC is using the case report form only during this acute outbreak response phase. CDC expects case data needs will decrease as we continue monitoring case count trends and understand more about how the virus is being transmitted.
Actions for Jurisdictions
Jurisdictions should send provisional case data as soon as they are available, even when they do not have complete information for all data elements in the case report form. CDC can receive partial case data and updates to previously sent data.
Although CDC released a fillable PDF version of the short case report form, jurisdictions should use electronic methods to send data elements included in the case report form to CDC.
Currently, jurisdictions can send mpox case report form data in multiple ways. These include:
- entering data directly into the Data Collation and Integration for Public Health Event Response (DCIPHER) platform,
- bulk uploading a CSV extract from a jurisdiction’s surveillance system into DCIPHER, and
- connecting a jurisdiction’s surveillance system to DCIPHER via application programming interface.
CDC has approval from the Office of Management and Budget to begin receiving mpox case data through routine NNDSS methods. Read more about transitioning case notifications to NNDSS data formats in the News You Can Use section below.
View the mpox short case report form and find more mpox information for health departments.
If you have questions about sending mpox data to CDC via NNDSS, email the CDC Electronic Data Exchange mailbox at edx@cdc.gov with the subject line “mpox reporting questions.”
An eSHARE webinar special session, “Provide Your Jurisdiction’s Feedback: Draft Brucellosis, Leptospirosis, and Hansen’s Disease Message Mapping Guides,” was held on July 26, 2022, 3:00-4:00 PM ET.
An eSHARE webinar, “Highlights from the CSTE 2022 Annual Conference.” was held on July 19, 2022.
Visit the eSHARE archives to access the slides and recordings for past eSHARE webinars. CDC typically posts eSHARE slides and recordings a few days after each webinar.
To join an eSHARE webinar, please see your calendar invitation or contact the CDC Electronic Data Exchange mailbox at edx@cdc.gov for login information with the subject line “eSHARE invitation.”
NEW! Tuberculosis and Latent TB Infection (LTBI) MMG
Version 3.0.3 of the TB and LTBI MMG has now posted. The changes include various formatting updates and updates to the test case scenario worksheet including two new LTBI scenarios.
Additionally, version 7 of the Tuberculosis and LTBI Case Notification View is now available. This view updates the value sets Medication (TB) and Susceptibility Test Type (TB) by correcting a spelling error in preferred concept name “Para-Aminosalicylic acid”:
- Value set Medication (TB) is used for data elements Drug Used to Treat MDR TB (INV1158) and Initial Drug Regimen (INV1143).
- Value set Susceptibility Test Type (TB) is used for data element Antimicrobial Susceptibility Test Type (LABAST6).
If you are interested in joining a cohort or onboarding an MMG, please email the CDC Electronic Data Exchange mailbox at edx@cdc.gov.
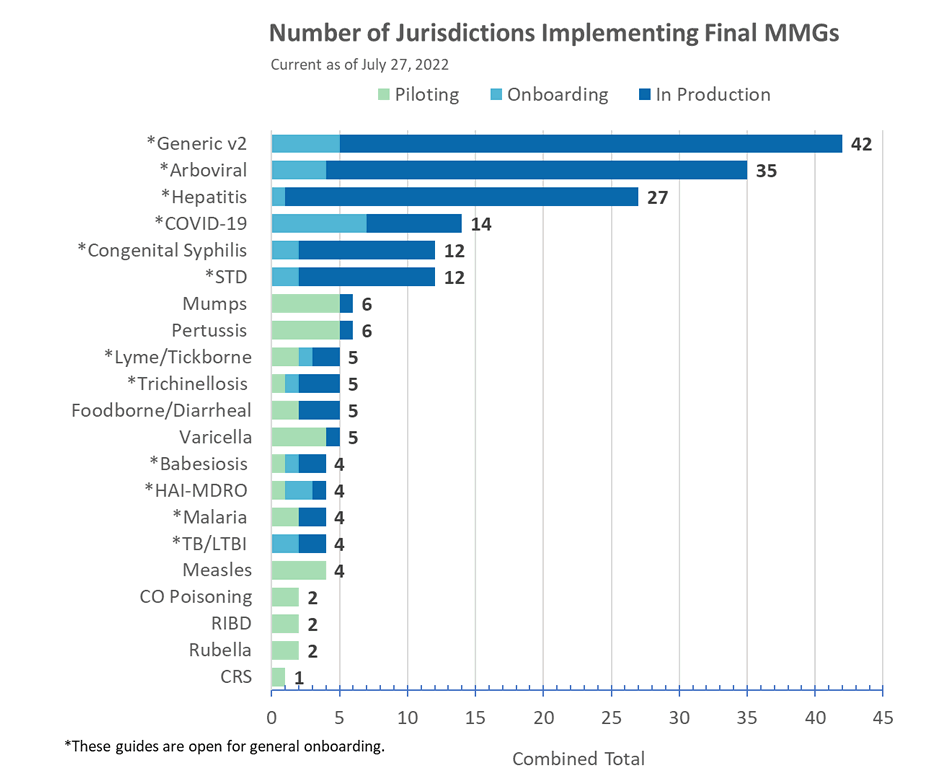
Congratulations to the following jurisdictions in production as of July 8, 2022!
- Indiana and Oregon for COVID-19 MMG
- New Mexico for GenV2 MMG (additional conditions)
Transitioning mpox case notifications to NNDSS
CDC has received approval from the Office of Management and Budget to begin receiving mpox case data using routine case notification methods.
CDC will post the mpox national surveillance case definition on the NNDSS case definitions webpage as soon as possible. Until that time, refer to the case definition in the Council of State and Territorial Epidemiologists (CSTE) position statement 22-ID-10.
Jurisdictions should start using the CSTE case definition described in the position statement on August 1, 2022. Jurisdictions should not retroactively change the classification of cases reported prior to August 1, 2022, based on the new case definition.
Ways jurisdictions can send mpox data to CDC through NNDSS include:
- HL7 Generic v2-based messages,
- National Electronic Disease Surveillance System Base System (NBS) Master Messages, and
- National Electronic Telecommunications System for Surveillance (NETSS) file format.
To initiate your NNDSS transmission, please email edx@cdc.gov with the subject “MPX validation.” NNDSS case notifications will be brought into DCIPHER, where they can be augmented with mpox-specific data sent through other methods. NNDSS case notifications will be an important method of updating case counts at CDC.
Learn more about transitioning mpox case notifications to NNDSS in the July 25, 2022 letter to State and Territorial Epidemiologists.
View the mpox short case report form and find more mpox information for health departments.
If you have questions about sending mpox data to CDC via NNDSS, email edx@cdc.gov with the subject line “mpox reporting questions.”