January 2023
February 21, 2023, 3:00–4:00 PM ET: February eSHARE Webinar: “Case Surveillance Data Standardization.”
June 25–29, 2023: 2023 Council of State and Territorial Epidemiologists (CSTE) Annual Conference.
Learn More.

Two NNDSS surveillance resources now available for 2023
To help jurisdictions participating in NNDSS conduct case surveillance, CDC’s NNDSS team posts event codes and other useful surveillance resources on the CDC website.
The team recently posted two new case surveillance resources for 2023:
- An updated list of Morbidity and Mortality Weekly Report (MMWR) weeks showing key reporting dates for NNDSS in 2022 and 2023. Learn more about MMWR
- A letter to State and Territorial Epidemiologists with an overview of changes coming to NNDSS in 2023.
CDC is planning to post additional resources for 2023 in the coming weeks. These include:
- list of all NNDSS event codes for 2023,
- PHIN VADS value sets for the 2023 event code list, and
- list of 2023 notification requirements.
CDC is also working to update our searchable library of case definitions based on 2023 position statements approved by CSTE.
Visit the NNDSS eSHARE archives for January 17, 2023 to get an overview of changes to NNDSS in 2023 and learn more about CDC surveillance resources for jurisdictions.
Have questions about case surveillance modernization?
Visit our Frequently Asked Questions page to find answers to common questions from state, local, and territorial jurisdictions.
Access archived NNDSS eSHARE webinars for monthly updates on case surveillance modernization.
Use this jurisdiction feedback form to provide feedback and ask questions.
The next eSHARE webinar, “Case Surveillance Data Standardization,” will be held February 21, 2023, 3:00–4:00 PM ET. The webinar will provide updates on work to standardize case surveillance data elements.
To join an eSHARE webinar, please see your calendar invitation or contact the CDC Electronic Data Exchange mailbox at edx@cdc.gov for login information with the subject line “eSHARE invitation.”
CDC is pausing most disease-specific (supplemental) message mapping guide (MMG) development and onboarding efforts. Learn more about how CDC is modernizing case surveillance. Get more information about CDC’s progress in this month’s News You Can Use section below.
NEW! Lyme and TBRD MMG
The PHIN Vocabulary Access and Distribution System (VADS) Lyme and Tickborne Rickettsial Diseases (TBRD) Case Notification View version 4 is now available for the Lyme and TBRD MMG. The changes in version 4 include:
- 15 additional values for Lab Test Type (Lyme), and
- Four additional values for Lab Test Type (TBRD).
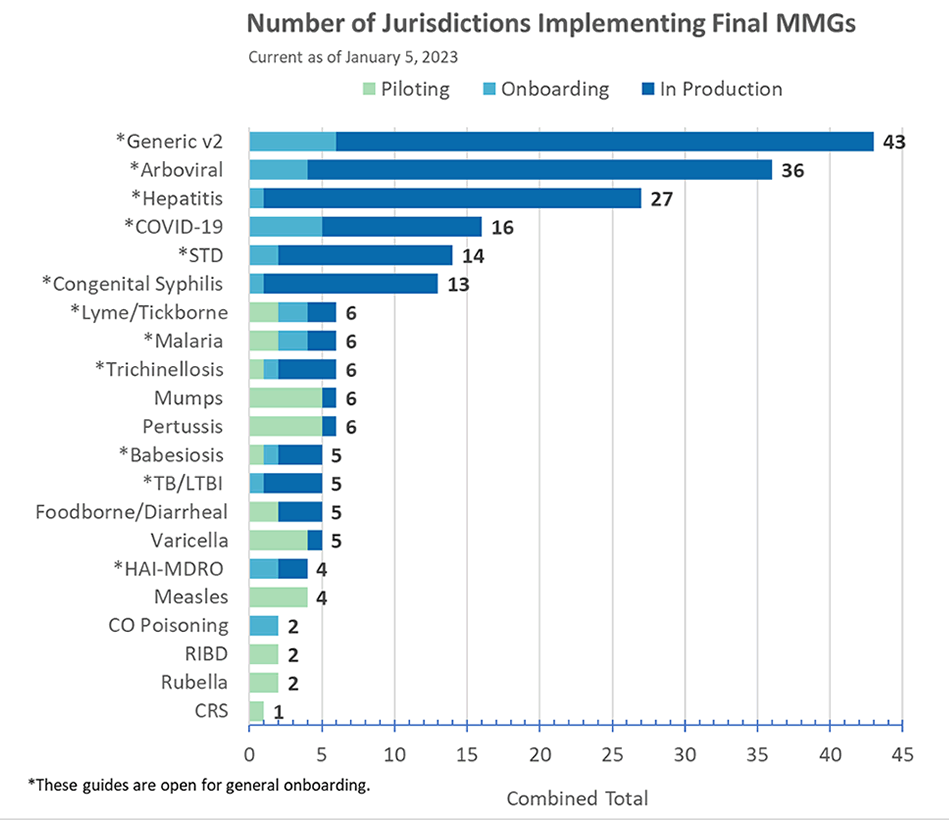
Congratulations to the following jurisdiction(s) in production as of January 5!
- Alaska and Utah for STD/CS MMGs
- Guam for Arboviral v1.3 MMG
- New York City for TB MMG
Case Surveillance Modernization Update
To enhance case surveillance and reduce burden on state, local, and territorial jurisdictions participating in the National Notifiable Diseases Surveillance System (NNDSS), CDC is collaborating with jurisdictions and public health partners to modernize case surveillance.
CDC is sharing recurring, operational progress updates through channels such as Case Surveillance News and monthly eSHARE training webinars. CDC will also share time-sensitive information about actions for jurisdictions, key milestones, and project decisions using the NNDSS email list via edx@cdc.gov.
Key implementation updates for January 2023:
- The MITRE landscape assessment to define barriers and inform solutions for sending GenV2 core data elements is now expected to begin in March 2023; however, the team is working to expedite required contract processes to try to begin the analysis before March.
- CDC is continuing to assess jurisdiction needs during the current pause on MMG implementation. This includes developing specific guidance for refocusing performance measures for the Epidemiology and Laboratory Capacity Health Information Systems (ELC HIS) Cooperative Agreement and the Emerging Infections Programs (EIP).
- The NNDSS team is continuing work with CSTE’s Data Standardization Workgroup and CDC Data Modernization Initiative priority teams to standardize an extended set of core data elements.
Jurisdiction support is critical to the success of this effort. CDC is continuing to work with jurisdictions to better understand their needs and status of MMG onboarding work. CDC encourages jurisdictions to give your input and feedback on any barriers or obstacles your organization may face with implementing the recommendations.
Visit CDC’s Frequently Asked Questions webpage to find answers to common questions from state, local, and territorial jurisdictions. Although we’re still working on the answers to many of your questions, CDC will continue updating this page as we have more information to share.
You can also use this jurisdiction feedback form to provide feedback and ask questions. We look forward to hearing from you!