NMI Notes

January 2021
Overall Updates
Holiday Greetings: We are grateful for your work and surveillance efforts in 2020! We wish you and your families a healthy and happy new year. We look forward to continuing our collaboration with you in 2021.
NNDSS Annual Data Reconciliation
- CDC closed the NNDSS database for updates to 2019 data on 12/10/20.
- CDC recognizes the demands of the response on jurisdiction and CDC resources and will not begin reconciling the 2020 data until late April 2021 at the earliest.
COVID-19 Case Surveillance Resources Available on NNDSS Webpage
- The updated COVID-19 case definition is available on the NNDSS Surveillance Case Definitions webpage.
- The COVID-19 message mapping guide (MMG) is available on the NNDSS Technical Resource Center. For those interested in implementing the COVID-19 MMG, please email the CDC Electronic Data Exchange mailbox at edx@cdc.gov.
- Case notifications for MIS associated with COVID-19 must be initiated in NNDSS. Use event code 11066. If a person meets the COVID-19 case definition and the MIS associated with COVID-19 case definition, CDC should receive separate case notifications for each condition using different Local Record IDs or National Electronic Telecommunications System for Surveillance (NETSS) Case IDs for each.
Core Requested Data Elements for COVID-19
- Due to the current high volume of cases, CDC is asking its state and local surveillance partners to focus efforts on a core set of data elements for COVID-19 case notifications, all of which can be sent using the generic v2 message.
- These data elements are based on information likely to be available from electronic laboratory reporting. CDC is asking states to focus on these core data elements but encourages states to send more as their resources allow. (For a list of data elements, please see slide 11 in the August 18 eSHARE presentation.)
- Jurisdictions are encouraged to send this information using the generic v2 message but may continue to use the legacy NNDSS messages or the CSV file upload to DCIPHER. For those interested in initiating generic v2 messages for COVID-19 and multisystem inflammatory syndrome (MIS) associated with COVID-19, please email the CDC Electronic Data Exchange mailbox at edx@cdc.gov.
- This does not change or reduce the data that state, territorial, and local jurisdictions choose to collect on their own for surveillance, public health investigations, or contact tracing.
- As resources allow, jurisdictions are encouraged to send additional case information through the CSV upload to DCIPHER or through the COVID-19 MMG. For those interested in implementing the COVID-19 MMG, please email the CDC Electronic Data Exchange mailbox at edx@cdc.gov.
NNDSS eSHARE Updates
- The next NMI eSHARE session is scheduled for Tuesday, 1/19/21, from 3:00 to 4:00 PM ET. The January eSHARE webinar will host a panel of states to expand upon the December 2020 eSHARE discussion on using immunization information systems to enhance disease surveillance and investigations during the response.
- Access past eSHARE presentations and details about future events at the NNDSS eSHARE website.
Join a Cohort to Jumpstart Your MMG Implementations!
- The NMI Association of Public Health Laboratories Technical Assistance team provides technical assistance to help groups, or cohorts, of public health agencies build and implement NNDSS HL7 case notification messages.
- Cohorts of 4–6 public health agencies focusing on the generic v2 and the COVID MMGs will be established.
- APHL and NEDSS Base System (NBS) subject matter experts guide participants through planning, gap analysis, data extraction, HL7 message creation, and validation. At completion, public health agencies will be ready to begin onboarding.
- For those interested in joining a cohort, please email the CDC Electronic Data Exchange mailbox at edx@cdc.gov.
Production HL7 Case Notification Messages
Arboviral v1.3
- The updated Arboviral MMG v1.3.1 has been posted on the NNDSS Technical Resource Center.
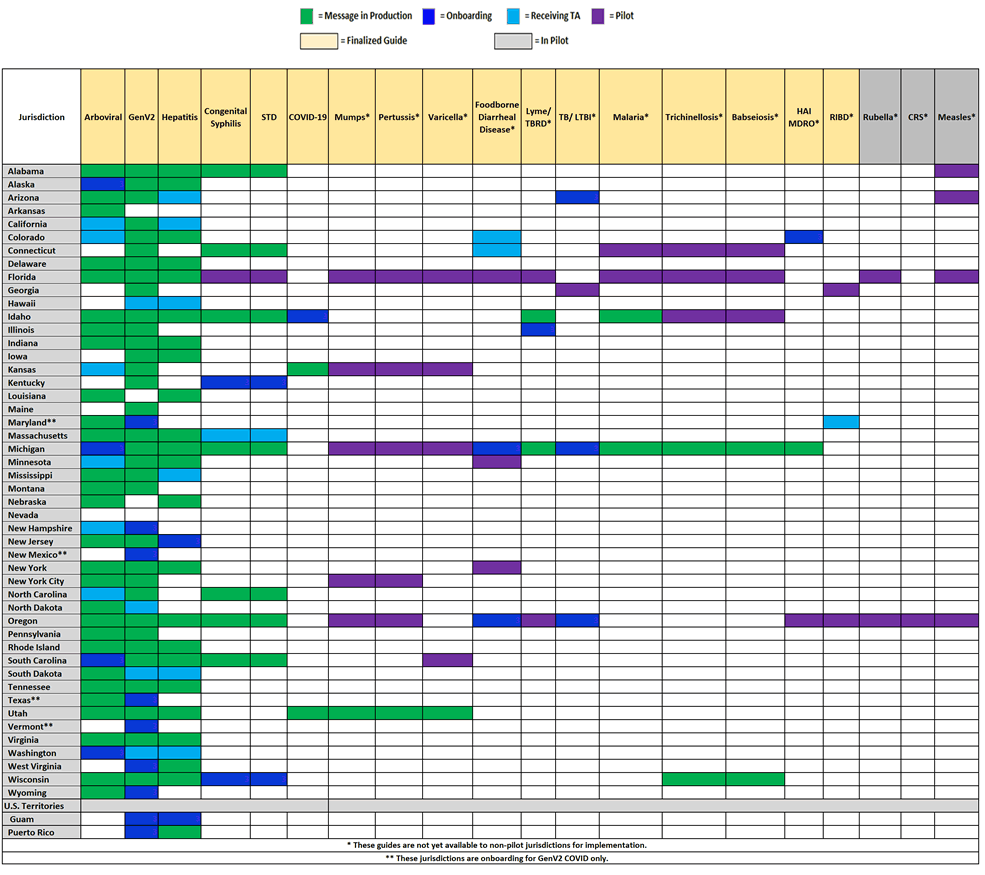
- There are 27 jurisdictions in production for arboviral.
- Alaska, Massachusetts, Michigan, South Carolina, and Washington are engaged in onboarding.
- Please see the chart below, Jurisdiction Implementation of NNDSS HL7 Case Notification Messages, for more details.
Babesiosis
- The MMG and artifacts are available on the NNDSS Technical Resource Center.
- Please use this guide to begin implementing your case notification messages in preparation for onboarding.
- There is 1 jurisdiction in production for babesiosis.
- Pilot jurisdiction Wisconsin is engaged in onboarding.
- Please see the chart below, Jurisdiction Implementation of NNDSS HL7 Case Notification Messages, for more details.
Congenital Syphilis and STD
- MMGs and artifacts are available on the NNDSS Technical Resource Center.
- CDC expects to post new versions of the guides, which include new data elements and updated value sets, to the NNDSS HL7 Case Notification Resource Center soon.
- Jurisdictions implementing current versions of the guides will be allowed to onboard with them.
- Jurisdiction users who have not yet implemented the current versions of the guides should wait until the new versions are released.
- Watch for an email with additional information, including guidance from the STD program on implementation of these guides.
- CDC is working with a set of states that have completed pre-onboarding work for the current versions of the guides.
-
- There are 6 pilot jurisdictions in production for STD and CS.
- Alabama, Kentucky, and Wisconsin are engaged in onboarding.
- Please see the chart below, Jurisdiction Implementation of NNDSS HL7 Case Notification Messages, for more details.
Coronavirus Disease 2019 (COVID-19)
- The MMG and artifacts are available on the NNDSS Technical Resource Center.
- The COVID-19 MMG is open for onboarding, and the first cohort has begun onboarding.
- Congratulations to Kansas, the first jurisdiction onboarded! Utah is engaged in onboarding.
- Please see the chart below, Jurisdiction Implementation of NNDSS HL7 Case Notification Messages, for more details.
Foodborne and Diarrheal Diseases
- The MMG and artifacts are available on the NNDSS Technical Resource Center.
- Pilot jurisdictions Oregon and Michigan are engaged in onboarding.
- Please see the chart below, Jurisdiction Implementation of NNDSS HL7 Case Notification Messages, for more details.
Generic v2
- The MMG and artifacts are available on the NNDSS Technical Resource Center.
- There are 33 jurisdictions in production for generic v2.
- Guam, New Hampshire, West Virginia, and Wyoming are engaged in onboarding.
- Please see the chart below, Jurisdiction Implementation of NNDSS HL7 Case Notification Messages, for more details.
Healthcare-Associated Infections, Multi-Drug Resistant Organisms (HAI MDRO)
- The MMG and artifacts are available on the NNDSS Technical Resource Center.
- Please use this guide to begin implementing your case notification messages in preparation for onboarding.
- There is one jurisdiction in productionfor HAI MDRO.
- Pilot jurisdiction Colorado is engaged in onboarding.
- Please see the chart below, Jurisdiction Implementation of NNDSS HL7 Case Notification Messages, for more details.
Hepatitis
- The MMG and artifacts are available on the NNDSS Technical Resource Center.
- There are 23 jurisdictions in production for hepatitis.
- Guam and New Jersey are engaged in onboarding.
- Please see the chart below, Jurisdiction Implementation of NNDSS HL7 Case Notification Messages, for more details.
Lyme and Tickborne Rickettsial Diseases (TBRD)
- The MMG and artifacts are available on the NNDSS Technical Resource Center.
- There are 2 jurisdictions in production for Lyme and TBRD.
- Pilot jurisdiction Illinois is engaged in onboarding.
- Please see the chart below, Jurisdiction Implementation of NNDSS HL7 Case Notification Messages, for more details.
Malaria
- The MMG and artifacts are available on the NNDSS Technical Resource Center.
- CDC expects to post minor updates to the MMG, artifacts, and an FAQ document by Spring 2021.
- There are 2 jurisdictions in production for malaria.
- Please see the chart below, Jurisdiction Implementation of NNDSS HL7 Case Notification Messages, for more details.
Mumps and Pertussis
- The MMG and artifacts are available on the NNDSS Technical Resource Center.
- There is 1 jurisdiction in production for mumps and pertussis.
- Please see the chart below, Jurisdiction Implementation of NNDSS HL7 Case Notification Messages, for more details.
Respiratory Invasive Bacterial Disease
- The MMG and artifacts are available on the NNDSS Technical Resource Center.
Trichinellosis
- The MMG and artifacts are available on the NNDSS Technical Resource Center.
- Please use this guide to begin implementing your case notification messages in preparation for onboarding.
- There is 1 jurisdiction in production for trichinellosis.
- Pilot jurisdiction Wisconsin is engaged in onboarding.
- Please see the chart below, Jurisdiction Implementation of NNDSS HL7 Case Notification Messages, for more details.
Tuberculosis and Latent Tuberculosis Infection
- The MMG and artifacts are available on the NNDSS Technical Resource Center.
- Pilot jurisdictions Arizona and Oregon are engaged in onboarding.
- Please see the chart below, Jurisdiction Implementation of NNDSS HL7 Case Notification Messages, for more details.
Varicella
- The MMG and artifacts are available on the NNDSS Technical Resource Center.
- There is 1 jurisdiction in production for varicella.
- Please see the chart below, Jurisdiction Implementation of NNDSS HL7 Case Notification Messages, for more details.
Developing HL7 Case Notification Messages
Bacterial Special Pathogens (Stage I—Draft)
- Prioritization of response activities has delayed the external review comment period to gather jurisdiction feedback.
- CDC expects to post the four MMGs (anthrax, brucellosis, Hansen’s disease, and leptospirosis) for external review in mid-2021.
Carbon Monoxide Poisoning (CO) (Stage II—Pilot Test-ready)
- The pilot start date has been postponed until March 2021.
Lead Poisoning (Requirements Analysis)
- No new updates at this time.
Listeriosis (Stage I—Draft)
- No new updates at this time.
Measles (Stage III—Final MMG)
- CDC anticipates posting the final guide and artifacts on the NNDSS Technical Resource Center in early 2021.
Rubella and Congenital Rubella Syndrome (Stage III—Final MMG)
- CDC anticipates posting the final guides and artifacts on the NNDSS Technical Resource Center in early 2021.
Resources
- NNDSS Technical Resource Center:
https://www.cdc.gov/nndss/trc/ - NNDSS Message Mapping Guides:
https://ndc.services.cdc.gov/message-mapping-guides/ - NNDSS eSHARE Monthly Webinar:
https://www.cdc.gov/nndss/trc/onboarding/eshare.html - NNDSS News:
https://www.cdc.gov/nndss/trc/news/
Jurisdiction Implementation of NNDSS HL7 Case Notification Messages
This chart shows the status of state public health departments who are piloting, receiving technical assistance, onboarding, or in production for HL7 case notification messages developed as part of the NNDSS Modernization Initiative.
Upcoming Events
- NNDSS eSHARE; 1/19/21, 3:00PM ET;
https://www.cdc.gov/nndss/trc/onboarding/eshare.html
NMI Notes provides monthly news updates about the National Notifiable Diseases Surveillance System (NNDSS) Modernization Initiative (NMI). It is a collaboration by the Centers for Disease Control and Prevention (CDC), Council of State and Territorial Epidemiologists (CSTE), and Association of Public Health Laboratories (APHL).
If a colleague forwarded this issue to you, we encourage you to subscribe at this link to ensure that you receive future issues of NMI Notes.
Write: edx@cdc.gov Visit: www.cdc.gov/nndss/
www.cste.org
Ready to request technical assistance or onboarding?
Please contact edx@cdc.gov for more information.
Have questions or feedback on NMI Notes?
Email edx@cdc.gov.