NSSP Update – April 2020

A Technical Newsletter for the NSSP Community of Practice | April 2020
Community of Practice
COVID-19 Slack Channel
A Slack channel has been created to facilitate real-time discussion among community members, NSSP staff, and other stakeholders working in support of the COVID-19 response. Some community members are using Slack to get feedback that they can use to improve queries whereas others are using the channel for concensus gathering. Slack is, essentially, a collaborative hub for sharing files and ideas in one searchable space. To join ongoing conversations about the coronavirus response, please email nssp@cdc.gov with the subject line “Add to COVID-19 Slack.”
Community Highlights

NSSP Community of Practice
- Held the March 2020 NSSP Community of Practice (CoP) call with a focus on syndromic surveillance during the COVID-19 response (check out recording here)
- Check out previous call recordings and other resources from the NSSP CoP here.
NSSP CoP Core Committee
- Postponed the in-person NSSP CoP Core Committee Meeting in response to the ongoing COVID-19 outbreak
Data Quality Subcommittee
- Hosted a presentation titled “A Surrogate for the Truth: Reexamination of North Dakota Traumatic Brain Injury Syndromic Data” with guest speakers Rasha Elnimeiry (ND), Kodi Pinks (ND), and Anastasia Stepanov (ND)
- Introduced the topic of processing data containing value sets during March 2020 Data Quality Subcommittee call
- Check out previous call recordings and other resources from the Data Quality Subcommittee here.
Knowledge Repository Curation Subcommittee
- Convened the leads of the subcommittee for the first time in March 2020 to develop a process for Knowledge Repository (KR) curation
- Be on the lookout for more updates from the KR Curation Subcommittee in the coming months.
Overdose Surveillance Subcommittee
- Hosted Mark Person (Pima Co., AZ) on the March 2020 Overdose Surveillance Subcommittee call to explain how his local health department uses different data sources for Opioid Crisis-related public health action
- Check out previous call recordings and other resources from the Syndrome Definition Subcommittee here.
Syndrome Definition (SD) Subcommittee
- Narrowed the scope of to-be-developed general mental health query—if you have thoughts or suggestions, please email syndromic@cste.org (Note: keep the query broad or focus on specific mental health outcomes, such as anxiety or depression)
- Presented on the CSTE Disaster Epidemiology Subcommittee to demonstrate the SD-developed Disaster-related mental health syndrome definition (Note: syndrome definition is available as a Chief Complaint [CC] and Discharge Diagnosis [DD] category in NSSP–ESSENCE and Knowledge Repository)
- Check out previous call recordings and other resources from the Syndrome Definition Subcommittee here.
Technical Subcommittee
- Cancelled Technical Subcommittee call scheduled for Thursday, April 2, 2020, at 2:00 PM ET
- Check out previous call recordings and other resources from the Technical Subcommittee here.

Spotlight on Syndromic Surveillance and Public Health Emergency Preparedness, Response, and Recovery (SPHERR) Subcommittee
SPHERR helps syndromic surveillance and public health preparedness professionals fully integrate syndromic surveillance data and information into emergency preparedness and response. SPHERR provides access to a national peer network for ad hoc support and collaboration during incidents and events of national interest (e.g., extreme weather and mass gatherings).
SPHERR Subcommittee
Co-Chair: Bill Smith (Maricopa County, AZ)
Co-Chair: Zach Faigen (NC)
Link to Meeting Resources on KR
If you’d like to join SPHERR, please email syndromic@cste.org.

Aaron Kite-Powell is a health scientist with the Division of Health Informatics and Surveillance, part of the Center for Surveillance, Epidemiology, and Laboratory Services.
Aaron Kite-Powell Recognized for Syndromic Work
The Department of Health and Human Services Gears of Government Award recognizes federal employees across the government who play pivotal roles in achieving the President’s vision for a modern, effective government that works on behalf of the American people. Health Scientist Aaron Kite-Powell, of CDC’s National Syndromic Surveillance Program, received this award for his work to improve the use of public health analytic and programming tools. Kite-Powel was recognized for his collaboration with state and local public health jurisdictions, public health partners, and analysts in other agency programs.
- NSSP Community of Practice (CoP) Landing Page and Calendar:
https://https://nsspcommunityofpractice.org/
CoP overview, description of workgroups and committees, call calendar and conference lines, and links to NSSP resources - Knowledge Repository (KR):
https://knowledgerepository.syndromicsurveillance.org/
Curated webinars, use cases, syndromes, stories of surveillance in action, meeting recordings, journal articles, and more

Look for an updated NSSP CoP website in Spring 2020!
ESSENCE Queries
February and March 2020
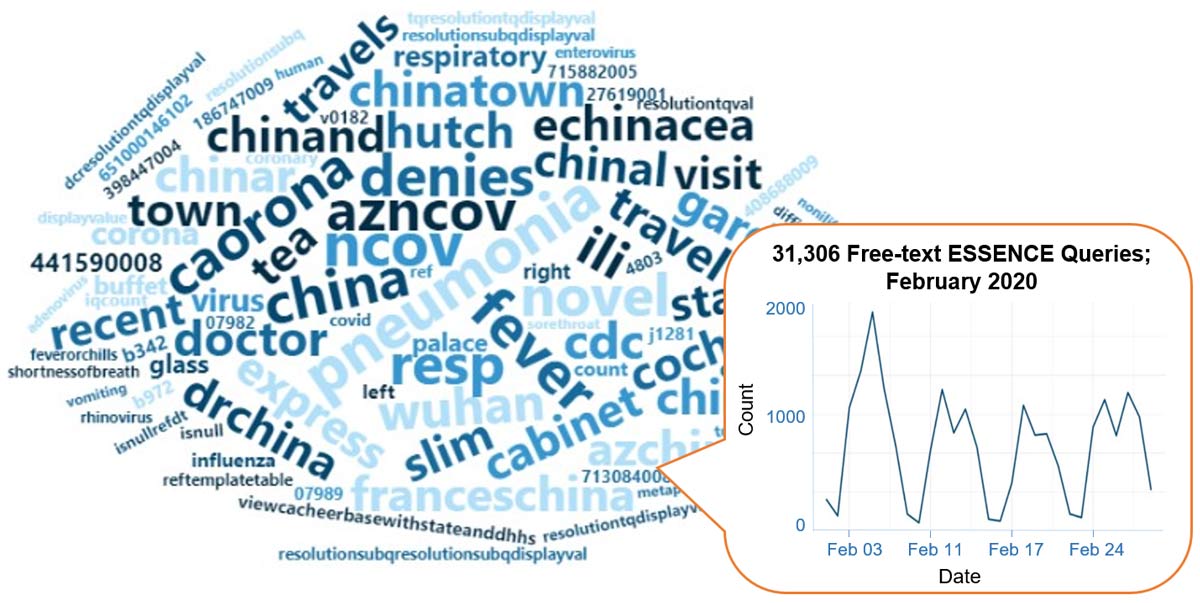
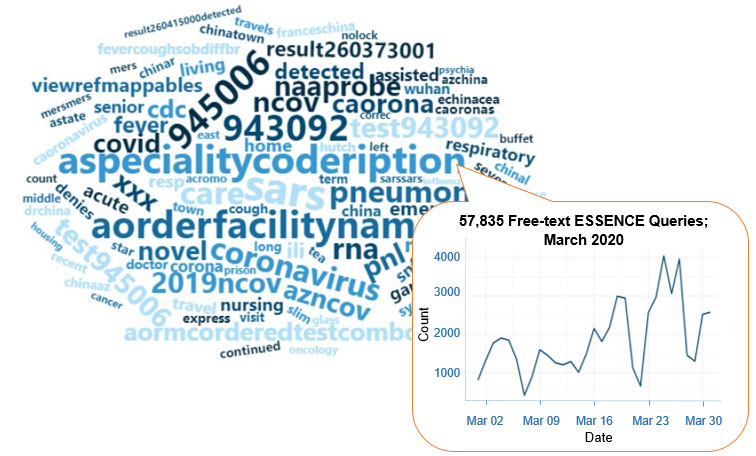
A key feature of NSSP–ESSENCE is its ability to use free-text definitions across different fields to generate custom queries. The syndromic surveillance community can use this feature to develop and refine category definitions and to identify and respond to public health events that may not be apparent in established categorical definitions (i.e., syndromes, subsyndromes, and Chief Complaint and Discharge Diagnosis [CCDD] categories).
This word cloud summarizes free-text and coded values that epidemiologists and data analysts queried in ESSENCE during February and March 2020. We created it by using the Wordcloud2 package in RStudio. The relative size of the terms suggests the frequency of use in a query. NSSP super administrators can query ESSENCE usage logs to learn what queries are run nationwide and how long they take to complete.
Questions and Tips
New and well-established sites continue to bring facilities onboard the BioSense Platform to improve data coverage. Here are some of the questions most frequently asked of the onboarding team:

Q: Why is it important to keep information in the Master Facility Table current during onboarding?
A: Everyone wants the onboarding process to progress smoothly and be error free. By updating the Master Facility Table (MFT) regularly and keeping it current, you decrease the likelihood for discrepancies once the facility begins transmitting data to NSSP. Any discrepancies between what the site administrator enters into the MFT versus what the facility sends could raise issues. For example, if the site administrator entered a FacilityID_UUID into the MFT different from that submitted in the data, the mismatched identifiers will prevent incoming data from mapping accurately to the facility listed in the MFT. Worse yet, data will not process and will continuously route to the exceptions table until the issue is resolved. (Tip: This is a good reason to check Data Quality reports in WinSCP for staging exceptions data or the Data Quality Dashboard and SAS Data Quality [DQ] On Demand for production exceptions data.)
Q: What does the NSSP onboarding team look for when they validate data quality?
A: NSSP’s approach to validating data quality provides two levels of minimum data quality requirements:
- Priority 1 Data Elements—Minimum Required Data Elements to onboard to NSSP BioSense Platform (compliance with PHIN Messaging Guide for Syndromic Surveillance); and
- Priority 2 Data Elements—Minimum Required Data Elements to comply with the Office of the National Coordinator for Health Information Technology (ONC) certification. ONC supports adoption of health information technology and promotes nationwide health information exchange to improve healthcare. See HealthIT.gov at https://www.healthit.gov/topic/about-onc.
NSSP encourages sites and facilities to achieve 100% compliance with data completeness, timeliness, and validation. Sometimes, however, considerable effort is needed to develop production-ready HL7 messages that fully comply with the messaging guide. Changes to messages can require considerable time and planning to implement correctly. Some nice-to-have changes might not be identified until late in the onboarding process, becoming prime candidates for integration into vendor software upgrades. Issues can also result from outdated documentation, vendor mergers, and user errors. Given these potential obstacles, having two levels of minimum data quality requirements is a practical solution.
Q: For whom are the onboarding guides and job aids designed, and what purpose do these documents serve?
A: The guides and job aids are designed to help site administrators onboard new facilities to the BioSense Platform and set up initial feeds in a manner consistent with NSSP guidelines. Facility managers, administrators, technical staff, vendors, health information exchanges, and individual hospitals or medical facilities that transmit syndromic data will also find the information useful. (For onboarding information specific to your local public health jurisdiction, please work directly with your site administrator.)
Q: Can sites submit personally identifiable information?
A: Please avoid—or limit—sending personally identifiable information (PII) to the BioSense Platform. NSSP can scrub PII from specific fields listed in the New Facility Onboarding Guide for the BioSense Platform. PII sent in other fields (those not listed) cannot be scrubbed and should not be sent. NSSP issues weekly PII reports to alert sites of problems and works with site administrators to identify solutions.
Q: What is the timeline for onboarding new facilities to the BioSense Platform?
A: The timeline will vary depending on facility and vendor readiness, facility connection to the data feed, and identification of issues throughout onboarding.
Once a new facility establishes a data flow to the onboarding environment, the site administrator may request a review of the data by submitting a ticket to the NSSP Service Desk or by changing the Facility Status on the Master Facility Table to Active:
- If no data quality issues are identified, the facility will usually be approved within 1 to 2 business days.
- If data quality issues are identified or facility prioritization is a concern, approval for production can take about 2 weeks. On average, 2 weeks are needed for the NSSP onboarding team to communicate the issue to the site administrator and for the site administrator either to resolve or respond to each problem.
Collaborations
COVID-19 Queries Added to ESSENCE
Fever and Cough-SOB-DiffBr v1—This query was created in collaboration with epidemiologist Natasha Close, Washington State Department of Health. It can be used to identify and monitor visits of persons who present with symptoms commonly seen among those who test positive for coronavirus. The free-text query efficiently searches the chief complaint discharge diagnosis (CCDD) category for visits binned into respective subsyndromes without degrading system performance.
Subsyndromes of interest: Fever or Chills and (Cough or Shortness of Breath or Difficulty Breathing)
Query: (,^;FeverorChills;^,and,(,^;Cough;^,or,^;DifficultyBreathing;^,or,^;ShortnessOfBreath;%,),)
- Fever and Cough-SOB-DiffBr neg Influenza DD v1—NSSP health scientists created this query to track visits with coronavirus-like symptoms while simultaneously negating (controlling) for visits with confirmed influenza. Confirmed influenza is identified by using discharge diagnosis codes (Influenza DD v1). The query then searches free text for visits binned into the appropriate subsyndromes. Binning this into a CCDD category allows BioSense Platform ESSENCE users to conduct analyses without negatively affecting system performance.
Query: (,^;FeverorChills;^,and,(,^;Cough;^,or,^;DifficultyBreathing;^,or,^;ShortnessOfBreath;^,),) and then also search CCDD Category Free Text for: isnull,or,^,andnot,(^influenza dd v1^,)

Collaborations between health departments and CDC programs* improve how syndromic surveillance gets done. The list of collaborations that follows, while not exhaustive, summarizes ongoing efforts to develop and improve syndrome definitions, conduct pilot tests and exercises, and improve processes to achieve public health goals more efficiently:
- Develop syndrome definition for pregnancy, delivery, and miscarriage.
- Collaborator: CDC’s Maternal and Infant Health/Maternal and Infant Health Branch
- THE SYNDROME DEFINITION IS COMPLETE! NSSP will inform the community when this definition is available for use and has been added to the Knowledge Repository.
- Develop syndrome definition for injecting drug users and endocarditis (infection of inner lining of heart chamber and valves).
- Collaborator: CDC’s National Center for Emerging and Zoonotic Infectious Diseases, Epidemiology Research and Innovations Branch
- Develop syndrome definition for motor vehicle collision syndrome.
- Collaborator: CDC’s Division of Unintentional Injury Prevention and the Transportation Safety Team
- THE SYNDROME DEFINITION IS COMPLETE! NSSP will inform the community when this definition is available for use and has been added to the Knowledge Repository.

- Develop syndrome definition for child abuse and neglect.
- Collaborator: CDC’s Division of Violence Prevention
- THE SYNDROME DEFINITION IS COMPLETE! NSSP will inform the community when this definition is available for use and has been added to the Knowledge Repository.
- Develop syndrome definition for child sexual abuse.
- Collaborator: CDC’s Division of Violence Prevention
- Develop syndrome definition for teen dating violence.
- Collaborator: CDC’s Division of Violence Prevention
- Develop syndrome definition for homeless populations.
- Collaborator: CDC’s Division of Violence Prevention
- THE SYNDROME DEFINITION IS COMPLETE! NSSP will inform the community when this definition is available for use and has been added to the Knowledge Repository.
- Develop syndrome definition for animal bites.
- Collaborator: CDC’s National Center for Environmental Health
- Develop syndrome definition for synthetic marijuana.
- Collaborator: CDC’s Opioid and Overdose Team
- Develop strategies to capture radiation exposures following a radiation disaster and examine whether syndromic surveillance can use poison control center data to supplement existing radiation exposure surveillance.
- Collaborator: CDC’s National Center for Environmental Health
- Assess whether snake bites increase after natural disasters and if data from NSSP and poison control centers can be incorporated to analyze snake bite incidence pre- and post-disaster.
- Collaborator: CDC’s National Center for Environmental Health
*The priorities driving these projects may change. Content is updated monthly to keep current with agency and program changes.
Technical Updates
Laboratory Data Availability

CDC’s Division of Health Informatics and Surveillance (DHIS), which manages NSSP, has implemented laboratory data in syndromic surveillance. DHIS currently receives data in near real time from five commercial clinical laboratory services, with additional labs being onboarded. These data represent lab tests ordered for reportable diseases and opioid drug use, and their associated results, and consist of about 300,000 HL7 messages per day.
The initiative was primarily funded with Opioid Overdose Emergency Response funds. Although the primary use case revolved around opioid overdoses, all data received are processed and made available to users regardless of disease or exposure category. Processes have been developed to ingest these data into ESSENCE for user query and analyses. Due to these efforts, the NSSP team has been able to respond quickly to the reporting and decision-support needs of the current emergency response. DHIS is also working with scientists at the Mayo Clinic to receive an HL7 feed to the BioSense Platform.
Additionally, the CDC Data Hub, which is also managed by DHIS, has acquired lab data from Quest Diagnostics to support emergency response. (Data Hub maintains multiple data sets for public health research.) Although the process is still under development, the plan is for Quest to transmit to Data Hub daily so that the BioSense Platform can “pick up” data and process into ESSENCE. DHIS has acquired another data source, BioReference, and is examining the feasibility of picking up data in a similar way. DHIS anticipates acquiring lab data from other commercial laboratories as well to augment this response.
How to Use Real-time Lab Tests for COVID-19
CDC is now receiving data from some commercial reference laboratories as part of the COVID-19 response and anticipates receiving data from additional labs in the coming weeks. Data include tests ordered for COVID-19, which are available before a result is received, in addition to both positive and negative results after tests are read.
Some of you may be thinking about how to access and use these data. The first step in accessing lab data is to contact us at nssp@cdc.gov and request a data receipt form. Sign and return the form, and NSSP will email you when access is approved.
Once you have access, you’ll be able to see maps, trends, and line-level data in an ESSENCE dashboard titled Lab Data for COVID19 Response, available in the Library. State and local health departments can use the dashboard to track orders separately for high-risk settings, such as nursing homes, and for dialysis and oncology clinics.
Periodically, NSSP staff will check for lab tests ordered by nursing homes and notify sites where the order was placed. NSSP will email notifications to whomever is listed on the data receipt form (e.g., site syndromic surveillance contacts, local healthcare-associated infections coordinators). You can change the recipients at any time. Please let us know if you find these emails useful.
Data Usage Note: The automated feeds from some labs transmit data to NSSP in real time. For this reason, positive lab results may appear in ESSENCE before state disease surveillance programs receive batched reports. Please do not contact labs directly to confirm ESSENCE data. Labs do not have the capacity to reconcile ESSENCE data with state reporting. You are always welcome, however, to contact nssp@cdc.gov if you have not received positive results shown in ESSENCE by the end of the day or if you notice systematic errors in the ESSENCE feed.
Server Upgrades
To support the COVID-19 response efforts, on March 23, 2020, the NSSP technical team upgraded all servers to improve capacity. Throughout the upgrade, the BioSense Platform continued to accept laboratory data.
New NSSP Publications

- BioSense Platform User Manual for Data-Quality-on-Demand Programs—The Data-Quality-on-Demand (DQOD) programs described in this manual offer real-time access to data quality measures and flexible ways to run reports. SAS Studio users can run data quality checks on their site data as needed and on their schedule. Published March 2020!
- BioSense Platform User Manual for SAS Studio—The manual explains how to configure your SAS account and efficiently use SAS Studio to connect to and analyze your site’s data. Also covered are the basic interface, features, navigation, and functionality. Scheduled for publication in April 2020!
Look for user manuals in the Technical Resource Center, Technical Publications and Standards.
Current Month and Upcoming Events
| April 21 | Vendor patches in Testing and Development environments: 6:00–10:00 AM ET |
| April 23 | Vendor patches in Onboarding and Production environments: 6:00–10:00 AM ET |
Last Month’s Technical Assistance
| March 4 | Made critical server patches to address vulnerability in Apache Tomcat |
| March 17 | Deployed vendor patches in Development and Testing environments |
| March 23 | Deployed vendor patches in Onboarding and Production environments |
| March 23 | Upgraded BioSense Platform servers to improve capacity for COVID-19 response |
Participation: The map below shows participation in NSSP as of March 31, 2020, with 59 sites (47 states and the District of Columbia).
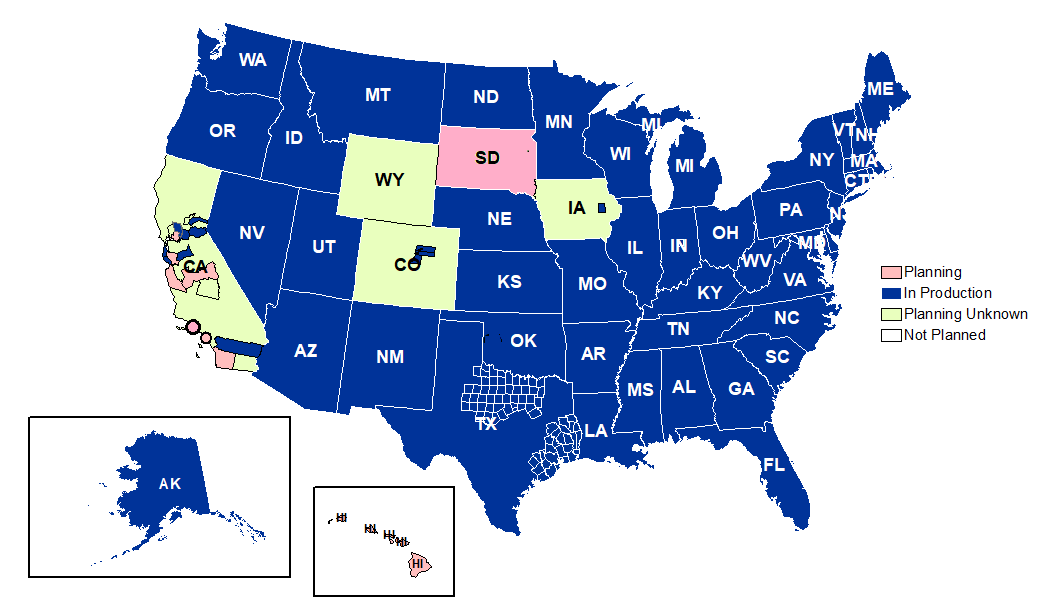
Definitions: NSSP consolidates facilities that provide data under a single data administrative authority called a site administrator. These facilities and single-site administrator constitute a site.
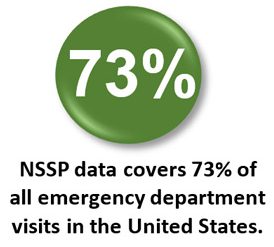
Coverage: NSSP publishes data for emergency department (ED) visit coverage each quarter. As of March 31, 2020, data from EDs covered about 73% of all ED visits in the country. There were 5,041 facilities, including 3,310 EDs from 59 sites (47 states and the District of Columbia), actively contributing data to the NSSP BioSense Platform. The calculation method is described in the December 2018 issue of NSSP Update.

