Technical Updates

A Look Back at 2020: Sites Respond to CDC’s Call to Action
2020 has been a uniquely challenging year. COVID-19 altered the focus on public health. CDC—with collaboration from NSSP participants nationwide—stepped up to meet new federal responsibilities affecting data sharing and to remain a source of high-quality data that supports public health decision-making.
Consequently, NSSP experienced many downstream effects, including a rapid increase in onboarding between March and December. In total, the NSSP onboarding team collaborated with site administrators, facility staff, and others to onboard 1,349 newly activated facilities in 2020. This far exceeds the number of facilities previously brought onboard during a 10-month period and improves data representativeness. NSSP participants are better equipped to conduct COVID-19 and other syndromic surveillance activities than ever before (Table 1).
|
 |
||||||||||||||||||||||||||
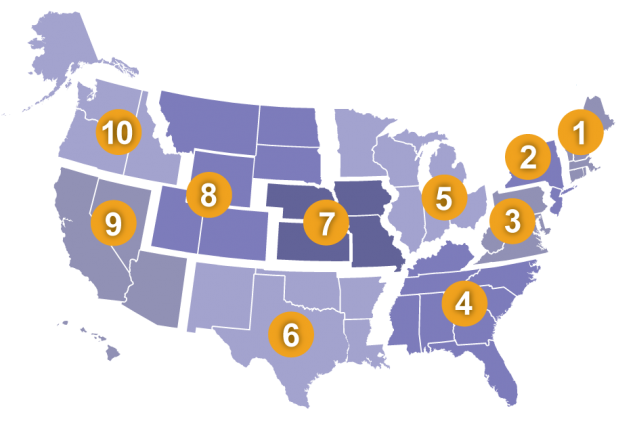
Participation in the fall 2020 New Site Onboarding Window far exceeded the previous window, which was held almost two years ago. Six new sites from regions 8, 9, and 10 participated this fall, whereas only one site completed the process last time. Each year, NSSP’s onboarding team assesses interest in onboarding and sets up Onboarding Windows as needed. If interested, e-mail nssp@cdc.gov.
The NSSP onboarding team would like to thank all site personnel and site administrators for their diligence and hard work this year. These efforts have helped tremendously with NSSP’s support of the COVID-19 response.
AMC Deployments, Developments, and Demos
In December 2020, the team completed the following activities in support of the Access & Management Center (AMC):
- deployed “Veterans Affairs Data Access” functionality to production;
- deployed “Master Facility Table (MFT)—Addition of New Sites” functionality to Production;
- performed internal demo of “Veterans Affairs Data Access” functionality;
- performed internal demo of “MFT—New Sites” functionality;
- performed internal demo of “Error Handling” functionality; and
- completed development of “Error Handling” functionality:
-
- completed quality assurance of “Error Handling” functionality and
- deployed “Error Handling” functionality to production.
 |
The rollout of COVID-19 vaccines heightens the need for situational awareness of people’s health after they get a vaccine. A new syndrome definition for monitoring side effects is now available: CDC Vaccine-Associated Adverse Events v1. |
Query Available to Help Identify Emergency Department Visits after Vaccination
On December 11, 2020, the Advisory Committee on Immunization Practices (ACIP) recommended the Pfizer-BioNTech COVID-19 vaccine for people 16 years of age and older in the United States. On December 19, 2020, the ACIP recommended the Moderna COVID-19 vaccine for people 18 years of age and older.
To make sure the benefits outweigh the risks for people who get vaccinated, several vaccine safety monitoring systems are in place to watch for adverse events (possible side effects). To further support these systems, state and local partners of the National Syndromic Surveillance Program (NSSP) have expressed interest in exploring the use of emergency department (ED) data to monitor safety.
In response, CDC NSSP developed and is sharing the first version of this query: CDC Vaccine-Associated Adverse Events v1. This query (shown below) is designed to identify mentions of adverse events after recent vaccination (VAE) during ED visits or in ambulatory healthcare settings. The query (syndrome definition) can be found in the ESSENCE chief complaint and discharge diagnosis (CCDD) category field.
The query includes a broad combination of diagnosis codes (ICD-10, ICD-9, and SNOMED) and chief complaint terms to detect visits potentially related to an adverse reaction after recent vaccination (e.g., poisoning, anaphylactic reaction, infection, post-vaccination fever, meningoencephalitis). This query does not attempt to identify specific clinical outcomes.
Given that discharge diagnosis can lag after chief complaint and is occasionally less complete, the NSSP team included chief complaint terms to improve timeliness and provide detailed reason-for-visit information. The chief complaint terms are designed to be more sensitive than specific and could identify ED visits unrelated to a recent vaccination or associated adverse event.
To join the group working on this and other queries or to ask questions about applying these searches to ED data in your public health jurisdiction, e-mail nssp@cdc.gov.
CDC Vaccine-Associated Adverse Events v1 Definition
Search CC field and also apply to DD field: (,(,^vaccin^,or,^immuniz^,or,(,^shot^,and,(,^ flu^,or,flu^,or,^influenza^,or,^shingles^,or,^pne^,or,^pna^,or,^tetn^,or,^tetanu^,or,^tdap^,or,^booster^,or,^td^,or,^hpv^,or,^mmr^,or,^covid^,or,^corona^,),),),and,(,^reaction^,or,^allerg^,or,^adverse^,or,^side effect^,or,^had^,or,^received^,or,^got^,or,^was given^,or,^after^,or,^post^,or,^last time^,or,^recent^,or,^since getting^,or,^since receiving^,or,^since yesterday^,),or,^shot^,AND,(,^Reaction^,or,^Allergic^,or,^Adverse^,),),andnot,(,^no vaccine^,or,^no immuniz^,or,^immunization not carried^,or,^[;/ ]Z28.21^,or,^[;/ ]Z2821^,or,^[;/ ]P55.1^,or,^[;/ ]P551^,or,^dog^,or,^cat^,or,^wound^,or,^up to date^,or,^ABO isoimmunization of newborn^,or,^well child^,or,^well visit^,or,^encounter for immunization^,or,^loss of consciousness^,or,^aftercare^,or,^UTD^,or,^up to date^,or,^bite^,or,^air vac^,or,^denies any previous adverse reaction^,or,^did not receive^,or,^poison ivy^,or,^allergic rhinitis^,or,^voiced no complaints^,),or,^[;/ ]T50[ABZ]^,or,^[;/ ]T50.[ABZ]^,or,^[;/ ]T80.52^,or,^[;/ ]T8052^,or,^[;/ ]T80.62^,or,^[;/ ]T8062^,or,^[;/ ]T88.[01]^,or,^[;/ ]T88[01]^,or,^[;/ ]R50.83^,or,^[;/ ]R5083^,or,^[;/ ]G04.32^,or,^[;/ ]G0432^,or,^[;/ ]G04.02^,or,^[;/ ]G0402^,or,^[;/ ]123471000119103[;/ ]^,or,^[;/ ]15920121000119100[;/ ]^,or,^[;/ ]219075006[;/ ]^,or,^[;/ ]219084006[;/ ]^,or,^[;/ ]293104008[;/ ]^,or,^[;/ ]293109003[;/ ]^,or,^[;/ ]293116002[;/ ]^,or,^[;/ ]293118001[;/ ]^,or,^[;/ ]293120003[;/ ]^,or,^[;/ ]293125008[;/ ]^,or,^[;/ ]31935007[;/ ]^,or,^[;/ ]408672009[;/ ]^,or,^[;/ ]47988006[;/ ]^,or,^[;/ ]219088009[;/ ]^,or,^[;/ ]293117006[;/ ]^,or,^[;/ ]420113004[;/ ]^,or,^[;/]97[89][;/ ]^,or,^[;/ ]97[89].[0-9][;/ ]^,or,^[;/ ]97[89][0-9][;/ ]^,or,^[;/ ]964.6[;/ ]^,or,^[;/ ]9646[;/ ]^,or,^[;/ ]999.0[;/ ]^,or,^[;/ ]9990[;/ ]^,or,^[;/ ]999.39[;/ ]^,or,^[;/ ]99939[;/ ]^,or,^[;/ ]999.[45]2[;/ ]^,or,^[;/ ]999[45]2[;/ ]^,or,^[;/ ]780.63[;/ ]^,or,^[;/ ]78063[;/ ]^,or,^[;/ ]323.5[;/ ]^,or,^[;/ ]323.5[12][;/ ]^,or,^[;/ ]3235[;/ ]^,or,^[;/ ]3235[12][;/ ]^
Date Created: 2020-12-10
Also see: CDC Website COVID-19 Vaccines and Severe Allergic Reactions
How to Encourage EHR Vendors to Send Data in Clinical Impression Field
The NSSP onboarding team has received questions from the community about the optional free-text field, Clinical Impression. Clinical Impression is considered an optional field in the PHIN Messaging Guide for Syndromic Surveillance 2.0. As an optional field, an electronic health record (EHR) vendor’s ability to populate this information largely depends on the system design. Not all EHR systems are designed to handle optional fields in the same way.
In the best-case scenario, the EHR vendor will be able to send the field with little additional work. If not, the EHR vendor might be able the send the free text after some development, research, or support.
Then again, the EHR may be
- unable to send the field in syndromic data at all;
- unable to send the field in this software version (may require an upgrade); or
- unable to send the Clinical Impression field but able to map that field to concatenate with another free-text field being sent.
States may require facilities to send a field that’s not required in the PHIN Guide. If an EHR vendor is unable to send Clinical Impression but the state requires inclusion of the free text in this field, facilities should be encouraged to discuss the requirement with their EHR vendor. EHR vendors should be able to send the free text in this field.
More questions? Contact nssp@cdc.gov.
New Data Elements Added to NSSP Data Dictionary
The NSSP Data Dictionary has been updated to include the latest additions to ESSENCE. We describe new data elements including HasBeenAdmitted, CCOrig_Length, CC_Parsed_Length, DD_Length, and MinutesFromVisitToDischarge, all of which are designed to assist with surveillance and assure data quality.
Additionally, we’ve added new demographic ESSENCE query parameters to the application programming interface (API) section. The current list of discharge disposition mappings after both “01 – Home/Self Care/Youth Home” and “01” were updated to DISCHARGED, while “A01” was removed from the mapping list.
Alternative to Adminer
Since retiring the Adminer tool in October, the onboarding team has received questions on RStudio and Structured Query Language (SQL). Most of these questions are answered with the reminder that all SQL queries previously used in Adminer can be run by following the instructions in the article How to do SQL Queries in RStudio. This article is a useful resource for those who need some guidance, and it has been updated since its initial publication to explain how SQL code can be copied into Microsoft Word. (Note: The code provided in the article requires the replacement of “curly” double quotes with double quotes once the code is copied into the R Studio window.)
Users who want to become familiar with R will find the information on the RStudio website helpful. See the RStudio Collections and Webinars web page.
NSSP Moves RStudio Pro to New Server
RStudio Professional Enterprise (RStudio Pro) edition has been moved to a new server and the R language module has been updated to the current 4.0.3 version.
During the move, existing shared projects were copied to the new server (see “Shared Projects” folder in User’s Directory) and properly archived. The old query server has been shut down and IP addresses reassigned to the new server.
This new configuration requires additional work to allow shared projects to function properly. In the close-out email sent December 21, 2020, for the transfer of RStudio Pro, we included instructions for reactivating transferred shared projects. If you didn’t receive these instructions, your site administrator should have a copy. If not, please open a service desk ticket.
Sharing a Project in RStudio (on the new server)

RStudio Pro lets you access and analyze SQL data stored on the BioSense Platform. You can use RStudio to verify data in the BioSense Platform archive (raw, processed, and exceptions tables), confirm information in your Master Facility Table (MFT), and view and query multiple databases and data tables. You can also create and share projects quite easily. Here’s how:
- First, you’ll need to create a new project in a new session in your home directory.
- In the top right corner, click the down arrow next to the new project name and select Share Project:
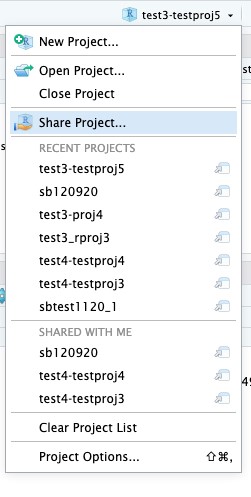
- Type the usernames of each person you want to share the project with. Then click the Add button.
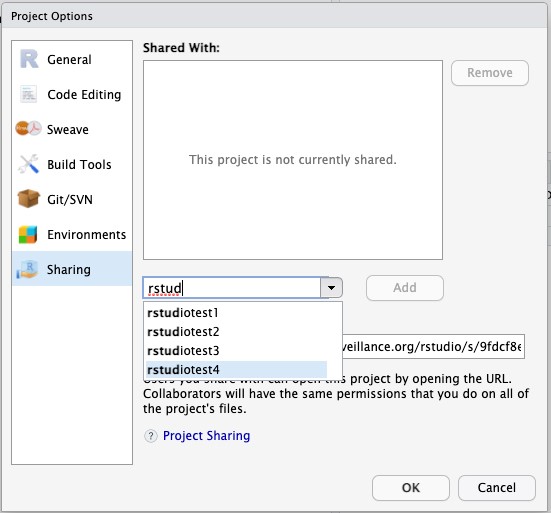
- Once you’ve added everyone with whom you want to share the project, click OK at the bottom of the dialog box.
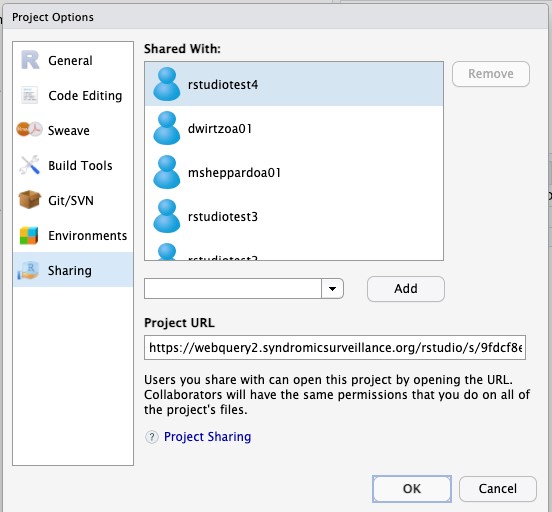
- You will receive the error message shown below. Take note of the file name at the end of the path. You’ll need to adjust the permissions on this file in the next step. Click OK.
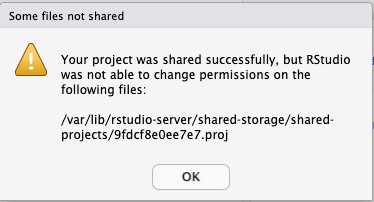
- In the left pane of RStudio, click on the Terminal tab. We are going to list the permissions for the files in the shared-projects metadata directory. Update the permissions on the new project that was shared. And then list the permissions again to verify that they were updated correctly.
- List the permissions by entering the ls -al /var/lib/rstudio-server/shared-storage/shared-projects/ command and pressing enter.
- Update the permissions on the new shared project by entering chmod g+rw /var/lib/rstudio-server/shared-storage/shared-projects/projectfile.proj. Make sure to change projectfile.proj to be the project file in the error message in Step 5. In this example, it is 9fdcf8e0ee7e7.proj.
- List the permissions to make sure the changes were successful, ls -al /var/lib/rstudio-server/shared-storage/shared-projects/.
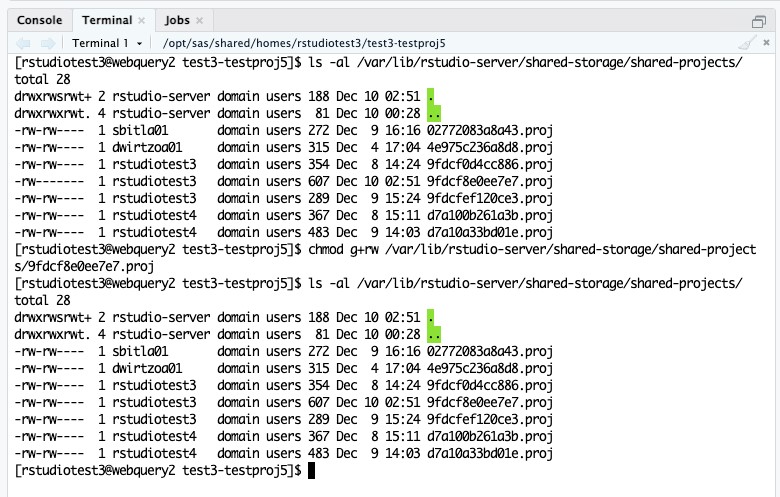
- The project is now accessible to everyone it was shared with.
