Technical Updates

NSSP Asks for YOUR Feedback on Proposal to Add CELR Data to ESSENCE
On February 1, 2021, the National Syndromic Surveillance Program (NSSP) emailed participants to describe a proposal for making data more comprehensive and representative. We proposed making coronavirus disease 2019 (COVID-19) electronic laboratory reporting (CELR) summary data available to ESSENCE users on the BioSense Platform and asked for your input on this proposal. A summary of the proposal follows:
The NSSP team at CDC has been working with the Applied Physics Laboratory at Johns Hopkins University on fusion models that combine data from multiple sources, including laboratory test results and emergency department visit data. These fusion models are designed to alert ESSENCE users to spikes in COVID-19 transmission at the county level. Currently, these models are built on a subset of data from six commercial laboratories that report directly to CDC through ESSENCE. The addition of CELR data will make this approach more comprehensive and representative, allowing better tracking of COVID-19 transmission and improving alerts to state and local authorities.
Data sent to ESSENCE would be a daily summary table generated of laboratory tests by county, result, age group (e.g., 0–4, 5–11, 12–17, 18–24, 25–34, 35–44, 45–54, 55–64, 65–74, 75+), and test type (PCR or antigen). CDC would generate the summary table, and no additional work is needed from you.
As with other data in ESSENCE, the NSSP site administrator in each state or county would determine state and local access to CELR data. The default view is for state users to have access only to data and alerts from their own state. Federal users would be granted access to CELR data in ESSENCE using the same access rules as those used for other systems, such as AIMS and HHS Protect.
Questions? Concerns? If you’re not interested in making your state’s CELR data available in ESSENCE, you may opt out by emailing both nssp@cdc.gov and edx@cdc.gov (electronic data exchange) by February 12, 2021.
ACEP’s Dashboard Helps Physicians Visualize ED COVID Data
The American College of Emergency Physicians (ACEP) has developed a dashboard that’s designed to help emergency department (ED) physicians see the impact of patient visits for COVID and influenza.
The dashboard uses national data drawn from CDC’s National Syndromic Surveillance Program to give users a new perspective on the concurrent effects of COVID and influenza. Users can see national data or drill down to see U.S. Health and Human Services (HHS) regional data (Figure 1). Updated weekly, the dashboard displays ED data across three categories: total visits, COVID-like illness visits, and influenza-like illness visits. ED physicians can immediately see regional changes for 7-, 30-, or 90-day periods; check trends; and get total visit counts.
This partnership among ACEP, CDC programs, and the NSSP Community of Practice demonstrates a novel use of NSSP data. High-level data can be publicly shared in a way that creates value for both the public and EDs—without compromising patient confidentiality.
See the dashboard on ACEP’s website.
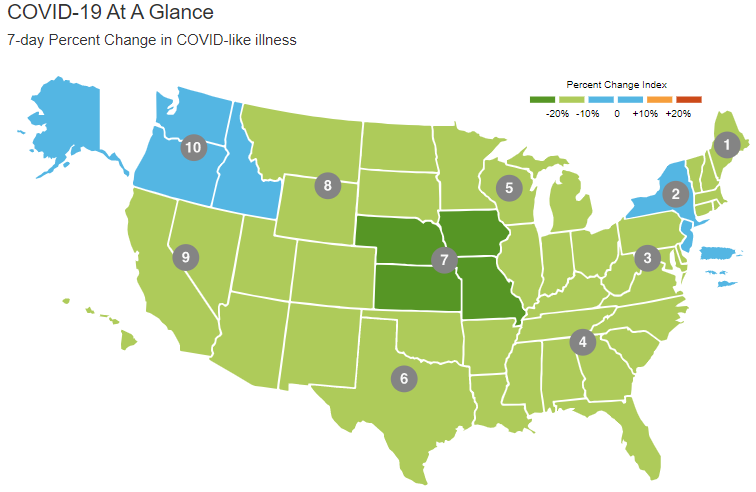
Figure 1. Shown above is U.S. emergency department data 7-day percent change in COVID-like illness by U.S. Health and Human Services region.
 |
| The rollout of COVID-19 vaccines heightens the need for situational awareness of people’s health after they get a vaccine. A new syndrome definition for monitoring side effects is now available: CDC Vaccine-Associated Adverse Events v1. |
Influenza Laboratory Keyword Syndromes Now Available

NSSP has developed new influenza laboratory test groupings (labeled “keyword syndromes” in ESSENCE) for users of the commercial laboratory data, Lab A. These groupings classify influenza test type by strain (All Flu, A, and B) and by results (all orders, all results, positive results, negative results, inconclusive results, test not performed, and antibody testing).
The advantage to having these groups is that you can quickly (and quite easily) extract Lab A influenza laboratory orders and results from the thousands of laboratory records in ESSENCE. The new test groupings are live on ESSENCE and available for analytic use.
New Syndrome Definitions and Updates
These new syndrome definitions are live on ESSENCE. The fact sheets will be added to the NSSP Community of Practice Knowledge Repository.
- CDC COVID-Specific DD v1,
- ILI Syndrome Neg Coronavirus DD v1,
- ILI CCDD Neg Coronavirus DD v1, and
- Intimate Partner Violence.
Updates have been made to CLI CC with CLI DD and Coronavirus DD v1, CLI CC with CLI DD and Coronavirus DD v2, and CDC Coronavirus DD v1.
Updated Query for COVID-19 Vaccine-Associated Adverse Event Surveillance
NSSP released the first version of a query designed to identify emergency department or ambulatory healthcare visits for adverse events after recent COVID-19 vaccination (VAE). This query (CDC Vaccine-Associated Adverse Events v1) can be found in the ESSENCE “CC and DD Category” field. It includes a broad combination of diagnosis codes (ICD-10, ICD-9, and SNOMED) and chief complaint terms to detect possible adverse events after recent vaccination (e.g., poisoning, anaphylactic reaction, infection, post-vaccination fever, meningoencephalitis). This query does not attempt to identify visits based on specific clinical outcomes; instead, it is designed to identify all visits after recent COVID-19 vaccination.
Two additional approaches can be combined with the Vaccine-Associated Adverse Events v1 query to allow evaluation of ED visits after vaccination for COVID-19:
- The following search string is designed to search for mentions of COVID-19 and the names of the currently approved COVID-19 vaccine manufacturers:
Code to apply Apply to chief complaint, chief complaint history/update, discharge diagnosis, triage notes, and clinical impression fields:
^Covid^,or,^Corona v^,or,^Coronav^,or,^modern^,or,^pfizer^,or,^phizer^ - The approach specified above can return a small number of visits for patients presenting with SARS-CoV-2 infections and histories of vaccination, rather than for examination for possible vaccine-associated adverse events. These visits can be excluded using the Coronavirus DD v1 query for a more specific look at adverse patient symptoms after the COVID-19 vaccine.
Code to search Search CC and DD Category Free Text:
^;CDC Vaccine-Associated Adverse Events v1;^,andnot,^;CDC Coronavirus-DD v1;^
These queries are designed to complement existing COVID-19 vaccine surveillance efforts. For more information on applying this query to ED data in your public health jurisdiction, e-mail nssp@cdc.gov.
NSSP Sites Begin Using Mortality Data
As of January 2021, two public health jurisdictions that participate in NSSP have mortality data in production NSSP–ESSENCE.
This is an exciting step after conducting a successful pilot of mortality data in 2020. During the pilot, public heath jurisdictions in Kansas, Oregon, and Washington State helped to refine data receipt, ingestion, and processing mechanisms; define data governance; and test the user interface.
So, how are mortality data processed? Mortality data are received in the standard Inter-Jurisdictional Exchange (IJE) electronic file format defined by the National Association for Public Health Statistics and Information Systems (NAPHSIS) IJE Committee. Jurisdictions can query Cause of Death codes. They can also query the Literal Cause of Death, a text field that provides the first indication of cause of death, which is like querying a chief complaint field in emergency department data. The Literal Cause of Death field is often received before the final ICD-10 codes, providing early insight and opportunity for timely analysis and response. Practitioners whose jurisdictions include mortality data in NSSP–ESSENCE will be able to integrate these data with illness, injury, and other health-related data in their response to public health threats and events.
If your public health jurisdiction is interested in onboarding mortality data and would more details, please contact nssp@cdc.gov.
NSSP Onboards its 49th State!
NSSP is proud to celebrate the successful onboarding of Wyoming, our 49th represented state. This addition completes U.S. Health and Human Services Region 8. In 2021, we look forward to meeting our next milestone—to represent all 50 states—as we work with public health practitioners in Hawaii. If you’re interested in seeing NSSP participation by U.S. county, click here.
Stay tuned for more updates, and, as always, email nssp@cdc.gov if you have questions.
AMC Update Scheduled for Spring 2021
The NSSP team continues to work on the BioSense Platform Access & Management Center (AMC) v1.5.4 software, scheduled for spring 2021 release. With this release, we’ll add the following features:
- time limits for data access rules that will allow users to specify the time and dates they want the data access rules to be active,
- ability to filter users by organization,
- enhanced messaging to provide descriptions of unmet requirements when changing passwords, and
- transaction management to reset failed updates when creating or changing data access rules or user groups.
Also, for users who manage data for the U.S. Department of Veterans Affairs, you’ll be able to further restrict data access by facility state and county.
 |
We need your help! We’re looking for volunteers to conduct user acceptance testing of AMC v1.5.4 software. Dates for the development release are February 16–24, 2021. If you’re interested but would like to see the test schedule before committing, contact nssp@cdc.gov. |
NSSP Fixes Software Issue
We are pleased to announce the production release of the BioSense Platform Access & Management Center (AMC) Version 1.5.3.5 software. With this release, we fixed an issue that prevented Clinical Laboratory Detailed Data from being displayed in ESSENCE.
This maintenance update was released to onboarding and production environments on Thursday, January 21, 2021.
More of Your Onboarding Questions Answered

Q. Some facilities that have been operational for years comply with “legacy” requirements but do not comply with the current standards in the PHIN Messaging Guide for Syndromic Surveillance 2.0. How can data from these facilities be improved?
A. The current standards in the PHIN Messaging Guide foster the use and exchange of consistent information among public health partners. This has become increasingly more important as health facilities modernize infrastructures to promote interoperability across public health systems. The PHIN Messaging Guide uses unique identifiers that allow HL7-compliant messaging and precise data storage. Transitioning facilities to the new message can improve the quality and completeness of data being transmitted. For assistance in review and improvement of production data, please submit an NSSP Service Desk Ticket.
Q. Is Patient Class a reliable indicator that data are from an emergency department facility? How does Patient Class compare with Facility/Visit Type or data listed on the Master Facility Table?
A. Facilities with emergency departments will often send inpatient or clinic data with a patient class of I or O—never having been E. These data (I or O) can be quite different from emergency department data. In the Master Facility Table (MFT), the Patient Class for the primary facility is simply listed as E with a Facility Type of Emergency Care. Because of this, the ‘HasBeenE’ data quality filter can filter patient visits that never had a Patient Class of E, removing all inpatient and clinic patients. Sometimes, however, the Patient Class is initially E but changed as the patient moves through the visit. For example, Patient Class could change from an initial E to an I when the patient gets an X-ray and to an O when getting laboratory tests. The NSSP onboarding team strives to ensure that the Facility/Visit Type in each message matches the Patient Class received and that these include the Patient Class and Facility Type noted in the MFT.
Q. Does ESSENCE have a data quality filter for Facility/Visit Type?
A. ESSENCE does not have a data quality filter for Facility/Visit Type. During onboarding, the NSSP team will confirm that the Facility/Visit Type listed on the Master Facility Table matches what’s contained in the incoming messages. They’ll also verify that the Patient Class being received matches the content of the incoming Facility/Visit Type.
