Technical Updates

NSSP-authored MMWRs Demonstrate the Value of Syndromic Surveillance
We’re committed to publishing important news about how NSSP data are being used by health practitioners, the research community, and public health educators. Last month we published three articles in CDC’s Morbidity and Mortality Weekly Report (MMWR):
- COVID-19 Pandemic–Associated Changes in Emergency Department Visits — United States,December 2020–January 2021: Emergency department visits during December 2020–January 2021 were 25% lower than during the same months the year before. Higher proportions of ED patients are seeking care for mental and behavioral health-related concerns, especially pediatric patients.
- Emergency Department Visits for COVID-19 by Race and Ethnicity—13 States, October–December 2020: Data from 13 states indicate that compared with White persons, Hispanic and American Indian or Alaska Native persons experienced 1.7 times the rate, and Black persons experienced 1.4 times the rate of emergency department care visits for COVID-19 during October–December 2020.
- COVID-19 Stats: COVID-19 and Influenza Discharge Diagnoses as a Percentage of Emergency Department (ED) Visits, by Year—United States, June 2018–March 2021
- Emergency Department Visits for Tick Bites—United States, January 2017–December 2019: A novel query of NSSP data indicated that one out of every 2,000 emergency department visits is for tick bites, with higher incidence during the spring and early summer and in the Northeast.
MMWR is CDC’s primary vehicle for scientific publication of timely, reliable, authoritative, accurate, objective, and useful public health information and recommendations. You can find more articles that show how NSSP data are being used here.
Updates Posted to Technical Resource Center
Check out these additions to the Technical Resource Center, Technical Publications and Standards:
- NSSP Data Dictionary—Update includes addition of new ESSENCE data elements (HoursFromVisitToDischarge, DaysFromVisitToDischarge), additions to ESSENCE API and Data Details tab, and addition of ESSENCE API Query Parameters tab.
- BioSense Platform User Manual for the Access & Management Center (AMC)—Revisions focus on the 1.5.4 release and include new data sharing parameters to specify dates when a data access rule will be active and what date range source data can be drawn from. Also, the Date and Time of the creation or last edit is displayed for new Data Access Rules in the listing on the Data Access tab.
Other enhancements mentioned in the updated user manual are that, on the Manage Users tab, the table of users can now be searched by organization and, on the Change Password dialog screen, password requirements are now tested as the user enters a new password. Each rule is displayed on the screen and checked off as the user enters a new password and fulfills each requirement, so, if all requirements are not met, the user will know which failed.
April 2021 Maintenance Update to AMC
The BioSense Platform AMC version 1.5.4.3 was installed on April 22, 2021. This maintenance release
- introduced additional traps for login errors and provided meaningful messages for users when these errors occur. This will
- reduce 404 and 500 browser errors and
- resolve blank screen on login error.
- resolved issue where user’s ESSENCE account was locked after the AMC password was changed.
- fixed Active/Inactive filter on the Reports tab so that it works as expected.
- corrected the problem that required users to change their browser zoom setting to activate the Rules of Behavior Accept button when resetting their passwords.
- resolved issues involving User Groups in Data Access Rules:
- User Groups can now be removed from a Data Access Rule.
- Abbreviated site name now shown on both the Available pick list and the Selected pick list.
- provided links to updated Community of Practice website (URL).
- resolved the issue where a site administrator couldn’t add a user to the rule because of this recurring error message: “An error occurred while updating user(s). Please try again and if the problem persists, contact the NSSP Help desk.”
- limited editing and deletion of user-created public user groups to the group’s site administrators and super administrators.
ESSENCE Laboratory Categorizations v1
The NSSP team has been working with subject experts at CDC to sort laboratory tests into categories, which is like sorting emergency department data into keyword syndromes. We’re pleased to announce that these categories are now available for use in ESSENCE and have the potential to significantly reduce processing time. Use these categories to quickly find laboratory tests for a specific pathogen, chemical, or other area of interest.
For this initial (beta) version of the look-up table, we identified all 1,398 unique laboratory test result codes within ESSENCE laboratory A data. To sort these test result codes into query-ready categories, we created a level 1 general category (drug test, respiratory disease test, etc.) that branches into a more refined level 2 category (opioid test, influenza test, etc.).
Next, we created 14 level 1 categories and 177 level 2 categories. Our level 1 categories include the following: Antibiotic and Other Antimicrobial Susceptibility, Blood Testing (Lead levels, parasites), Cancer Screening, Drug Testing, Gastrointestinal Infection, Hepatitis, Infectious Disease, Microbial Identification Testing, Respiratory Disease, Sexually Transmitted Disease/Infections, and Vector-Borne Diseases. Examples of the level 2 categories, which are too extensive to list here, range from Antibiotic Susceptibility for Azithromycin, penicillin, or Tetracycline to “Drug Testing: Opioid” and “Respiratory Disease: Pneumonia.” Depending on the health jurisdiction’s medical laboratory ordering practices, not all test categories will be present for all states. For example, if doctors in a northern health jurisdiction do not order tick bite tests, the results for tick bite tests will not show up for that state.
Each test result will belong to one and only one level 1 and to one and only one level 2 category. Conversely, each laboratory specimen could have multiple categories assigned depending on how many specimen tests were ordered. Put differently, you can select more than one category without the risk of counting the test result twice.
You can check out these test result categories for queries in the Laboratory by Result table in ESSENCE. Meanwhile, we are working with laboratory A to make the backend lookup table available for public use. We thank our partnering subject experts across CDC for their review of these categories. We encourage you to provide feedback or ask questions about this version by emailing nssp@cdc.gov.
If you found this article helpful, you might like last month’s article on “New ESSENCE Table Shows Laboratory Data by Individual Results.”
Notable Changes to CDC COVID-19 Data Tracker
The widespread adoption of the International Classification of Diseases, 10th Revision (ICD-10) codes for COVID-19 lets analysts query data by discharge diagnosis (DD) codes. As a result, diagnostic code-based queries are valuable and have fast become analysts’ primary tool for generating easy-to-understand visuals that communicate complex concepts (e.g., “dashboards”). The April 2021 release of CDC COVID Data Tracker reflects NSSP’s improvements to the DD-only query that will help analysts readily identify COVID-19 trends in emergency department (ED) visits.
NSSP’s improvements to the visuals focus on diagnosis, not symptoms. This is done by using the CDC COVID-specific DD v1 syndrome definition to limit the display of national, regional, and state-level trends to ED visits associated with the COVID-19 diagnosis and by removing coronavirus-like illness and other options. This approach provides a more accurate representation of ED visits associated with COVID-19. In addition, NSSP has extended the viewable timeframe of the visualization to include data through May 1, 2020. The extended period will help analysts assess trends over time and makes the presentation of ED data consistent with other surveillance data sources used by CDC’s COVID Data Tracker.
COVID Data Tracker will soon display trends in ED visits by age groups. This visual will improve our perspective of patients who present with COVID-19 in EDs. Like the April 2021 improvements, this visual will use the CDC COVID-specific DDv1 syndrome definition and include national, regional, state, and age group stratifications.
We thank the syndromic community for their thoughtful and consistent feedback to improve the quality and presentation of syndromic data.
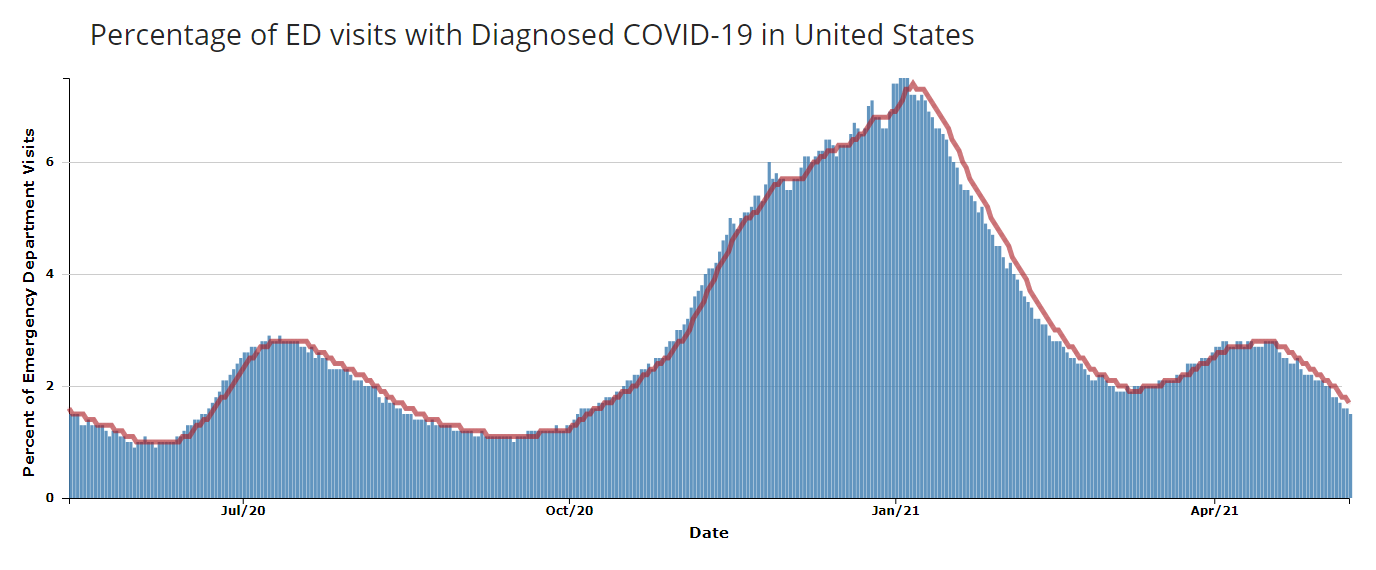
Shown above is an excerpt from the April 2021 update to CDC’s COVID Data Tracker that draws from NSSP data. COVID Data Tracker provides surveillance data from across the response, including hospitalizations, vaccinations, demographic information, and daily and cumulative case and death counts reported to CDC. For an interpretive summary of the week’s events and downloadable data, visit COVID Data Tracker Weekly Review.
