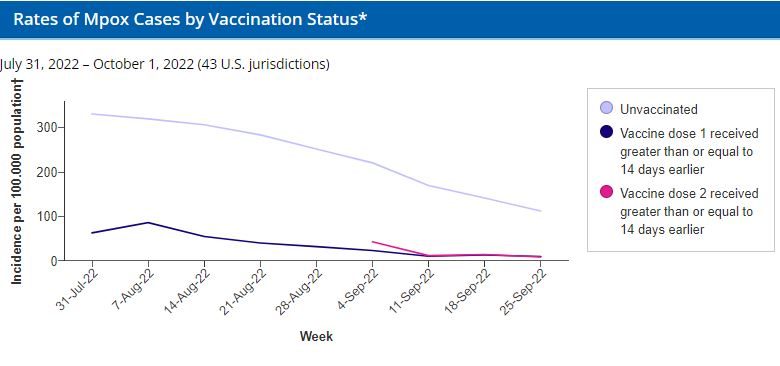
These data were posted on December 8, 2022 and reflect confirmed and probable mpox cases with illness onset between July 31, 2022 through October 1, 2022. Please note that these provisional data are subject to change.
*Vaccination Status: Unvaccinated: no evidence in case record of receipt of vaccine or vaccination date before illness onset, including records where vaccination information was unknown; vaccinated, illness onset 14 days or more after dose 1: illness onset 14 days or more after receiving dose 1 of vaccine, excluding people vaccinated for smallpox before 2022 and people who received dose 2 of vaccine; vaccinated, illness onset 14 days or more after dose 2: illness onset 14 days or more after receiving dose 2 of vaccine, excluding people vaccinated for smallpox before 2022. Linkage of mpox case surveillance and vaccination administration data might have resulted in misclassifications.
†Population: The population aged 18-49 years eligible for vaccination is derived from the estimated size of the underlying population in each jurisdiction that might benefit from expanded vaccination in the context of the outbreak and includes gay, bisexual, and other men who have sex with men (MSM) with HIV or who are eligible for HIV preexposure prophylaxis (HIV-PrEP), according to Phase 4 of the National mpox Vaccine Strategy (Administration for Strategic Preparedness & Response). To estimate the number of MSM with HIV, jurisdiction-specific estimates of the prevalence of HIV in 2020 from CDC Atlas Plus describing MSM who acquired HIV from the following transmission categories: “male-to-male sexual contact” and also “male-to-male sexual contact and injection drug use” were used. To estimate the number of MSM who are HIV-PrEP-eligible, the number of people in each jurisdiction prescribed HIV-PrEP, HIV-PrEP coverage in each jurisdiction, the percent of people on HIV-PrEP who are men nationally, the national HIV-PrEP coverage among MSM, and the national HIV-PrEP coverage among men and women from CDC Atlas Plus were combined. The number of MSM with HIV or who are eligible for HIV-PrEP aged 18–49 years was estimated by aggregating 2021 U.S. Census Bureau estimates for males aged 0–12, 13–17, 18–49, and ≥50 years, calculating the state proportion in each age group, and multiplying by the estimated number of MSM with HIV or who are eligible for HIV-PrEP in each state to obtain proportional distributions.
Mpox Case: A confirmed case of mpox is defined by presence of mpox virus DNA by polymerase chain reaction (PCR) testing or Next-Generation sequencing of a clinical specimen OR isolation of mpox virus in culture from a clinical specimen. A probable case is defined by presence of orthopoxvirus DNA by PCR, or orthopoxvirus using immunohistochemical or electron microscopy or detectable levels of anti-orthopoxvirus IgM antibody.
Participating jurisdictions: Currently, the following 43 health departments identify vaccination status through case interview or link case surveillance data to their own immunization registries and separately submit de-identified vaccine administration data to CDC and are included in these incidence rate estimates: Alabama, Alaska, California, Colorado, Connecticut, District of Columbia, Florida, Georgia, Hawaii, Idaho, Illinois, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nevada, New Hampshire, New Mexico, New York (excluding New York City), North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Puerto Rico, Rhode Island, South Carolina, South Dakota, Tennessee, Utah, Vermont, Virginia, West Virginia, Wisconsin, and Wyoming. The data assessed from 43 jurisdictions accounted for 72% of the U.S. population eligible for vaccination.
Illness Onset: Illness onset date refers to the earliest date available for a case. The selection of illness onset dates varied by how the case was reported into the system and may have been dates of any of the following (listed in order by priority and availability): illness onset, specimen collection, lab test completion, admission, diagnosis, discharge, initiation of case investigation, or first electronic submission, or report to the county, state, or public health department.
Incidence rate estimates: Weekly incidence measures were calculated by vaccination status as the number of cases divided by the number of people either unvaccinated as of that week or vaccinated 14 days or more prior to that week.
Incidence rate ratios (IRR): The incidence rate ratio (IRR) during the study period was estimated using negative binomial regression, adjusting for week in the model.
Publications:
- Payne AB, Ray LC, Kugeler KJ, et al. Incidence of Mpox Among Unvaccinated Persons Compared with Persons Receiving ≥1 JYNNEOS Vaccine Dose — 32 U.S. Jurisdictions, July 31–September 3, 2022. MMWR Morb Mortal Wkly Rep 2022;71:1278–1282. DOI: http://dx.doi.org/10.15585/mmwr.mm7140e3.
- Payne AB, Ray LC, Cole MM, et al. Reduced Risk for Mpox After Receipt of 1 or 2 Doses of JYNNEOS Vaccine Compared with Risk Among Unvaccinated Persons — 43 U.S. Jurisdictions, July 31-October 1,2022. MMWR Morb Mortal Wkly Rep. ePub: 8 December 2022. DOI: https://www.cdc.gov/mmwr/volumes/71/wr/mm7149a5.htm?s_cid=mm7149a5_w