National Typhoid and Paratyphoid Fever Surveillance Annual Summary, 2015
National Typhoid and Paratyphoid Fever Surveillance
Read an overview of the National Typhoid and Paratyphoid Fever Surveillance (NTPFS) system [PDF – 2 pages]
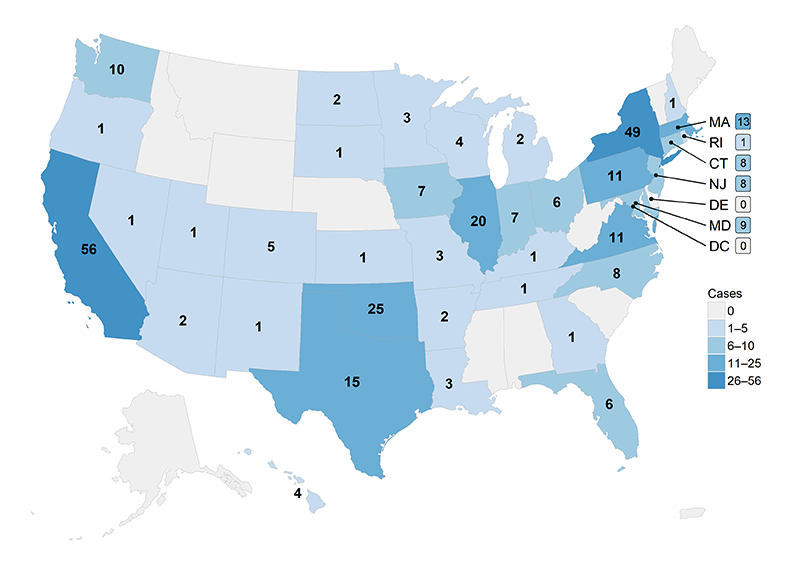
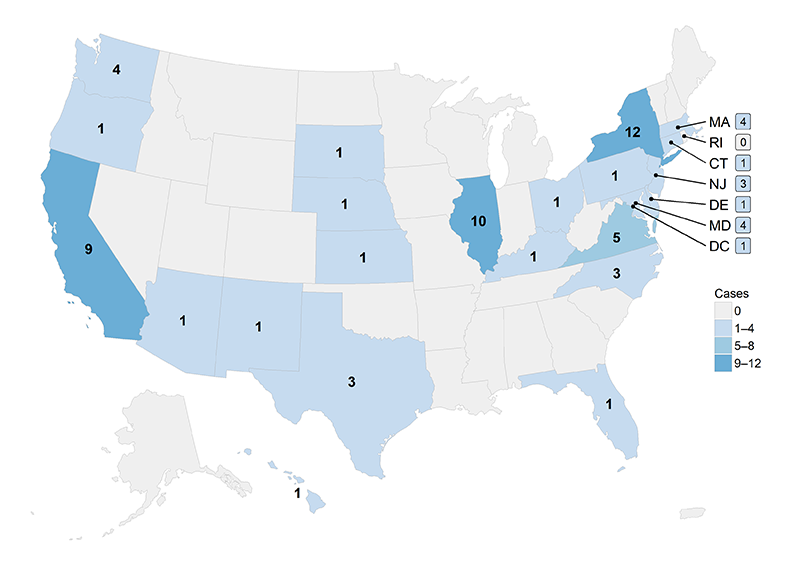
Jurisdictions1 reporting at least one typhoid or paratyphoid fever2 case to the NTPFS during 2015 are shown in Figures 1 and 2.
- 38 jurisdictions reported 309 typhoid fever cases (Figure 1).
- 24 jurisdictions reported 71 paratyphoid fever cases, all of which were caused by Salmonella serotype Paratyphi A (Figure 2).
1Includes all 50 states and District of Columbia
2Paratyphoid fever is caused by Salmonella serotypes Paratyphi A, Paratyphi B, and Paratyphi C. Two distinct pathotypes of Paratyphi B are recognized; one is associated with paratyphoid fever and the other is associated with uncomplicated gastroenteritis. The two pathotypes have distinct virulence characteristics, and are differentiated based on the ability to ferment tartrate. The paratyphoidal pathotype is unable to ferment tartrate and is designated serotype Paratyphi B; the nonparatyphoidal pathotype ferments tartrate and is designated serotype Paratyphi B var. L(+) tartrate+. Only those isolates laboratory-confirmed as not able to ferment tartrate are included in the annual NTPFS summary. For many Paratyphi B reports submitted to CDC, this information is not available; these reports are therefore excluded from the NTPFS summary.
Demographic and Clinical Characteristics of Patients
Demographic and clinical characteristics of patients with typhoid fever and paratyphoid fever are shown in Tables 1 and 2.
- The median age of patients with typhoid fever was 23 years (range 0–79).
- The median age of patients with paratyphoid fever was 29 years (range 1–79).
- There were no deaths from typhoid fever or paratyphoid fever.
Demographic and clinical characteristics of patients with typhoid fever reported to National Typhoid and Paratyphoid Fever Surveillance, 2015 (n = 309)
| Characteristic | Count | Percent |
|---|---|---|
| Age Group (n = 307) | ||
| <1 | 2 | <1 |
| 1–4 | 31 | 10 |
| 5–14 | 76 | 25 |
| 15–24 | 57 | 19 |
| 25–44 | 112 | 37 |
| 45–64 | 23 | 8 |
| 65+ | 6 | 2 |
| Female (n = 306) | 144 | 47 |
| Food handler (n = 172) | 6 | 3 |
| Outbreak-associated (n = 276) | 42 | 15 |
| International travel 1 (n = 303) | 240 | 79 |
| Vaccinated2 (n = 178) | 5 | 3 |
| Specimen source (n = 305) | ||
| Blood | 264 | 87 |
| Stool | 34 | 11 |
| Other | 7 | 2 |
| Hospitalizations3 (n = 295) | 238 | 81 |
| Deaths (n = 293) | 0 | 0 |
1Travel destinations are shown in Table 3.
2Received typhoid fever vaccination within 5 years before onset of illness; of the five cases in vaccinated persons, two received only the oral, live attenuated vaccine (Ty21a), one received the Vi capsular polysaccharide vaccine (ViCPS) and the oral, live attenuated vaccine, and the vaccine type was not reported for the other two.
Demographic and clinical characteristics of patients with paratyphoid fever reported to National Typhoid and Paratyphoid Fever Surveillance, 2015 (n = 71)
| Characteristic | Count | Percent |
|---|---|---|
| Age Group (n = 70) | ||
| <1 | 0 | 0 |
| 1–4 | 4 | 6 |
| 5–14 | 14 | 20 |
| 15–24 | 9 | 13 |
| 25–44 | 29 | 41 |
| 45–64 | 10 | 14 |
| 65+ | 4 | 6 |
| Female (n = 71) | 34 | 48 |
| Food handler (n = 35) | 0 | 0 |
| Outbreak-associated (n = 61) | 4 | 7 |
| International travel 1 (n = 66) | 60 | 91 |
| Specimen source (n = 69) | ||
| Blood | 55 | 80 |
| Stool | 9 | 13 |
| Other | 5 | 7 |
| Hospitalizations2 (n = 68) | 48 | 71 |
| Deaths (n = 66) | 0 | 0 |
1Travel destinations are shown in Table 3.
International Travel
- Among the 303 typhoid fever patients whose travel histories were reported, 240 (79%) reported traveling or living outside the United States in the 30 days before illness and 63 (21%) reported no travel.
- Among the 66 paratyphoid fever patients whose travel histories were reported, 60 (91%) reported traveling or living outside the United States in the 30 days before illness and 6 (9%) reported no travel.
- Among patients reporting international travel, 223 (93%) patients with typhoid fever and 57 (95%) patients with paratyphoid fever reported travel to a single destination (Table 3).
- Visiting friends or relatives was the most common reason for international travel among patients with typhoid fever (54%) and paratyphoid fever (52%).
Travel destinations for patients with typhoid fever who reported a single destination country, National Typhoid and Paratyphoid Fever Surveillance, 2015
| Travel Destination | No (%) |
|---|---|
| India | 131 (59) |
| Pakistan | 23 (10) |
| Bangladesh | 22 (10) |
| El Salvador | 8 (4) |
| Mexico | 8 (4) |
| Other1 | 31 (14) |
1Patients reported travel to Haiti (5), Guatemala (3), Philippines (3), Samoa (3), Ecuador (2), Nigeria (2), Angola (1), Barbados (1), Burma (1), Cambodia (1), Chad (1), Colombia (1), Costa Rica (1), Honduras (1), Iraq (1), Kenya (1), Tanzania (1), Uganda (1), United Arab Emirates (1).
Travel destinations for patients with paratyphoid fever who reported a single destination country, National Typhoid and Paratyphoid Fever Surveillance, 2015
| Travel Destination | No (%) |
|---|---|
| India | 30 (53) |
| Pakistan | 14 (25) |
| Bangladesh | 4 (7) |
| Burma | 1 (2) |
| Cambodia | 1 (2) |
| Other1 | 7 (12) |
1Patients reported travel to Canada (1), China (1), Nepal (1), Other (1), Senegal (1), Thailand (1), United Arab Emirates (1).
Typhoid Fever Vaccine
Figure 3. Percentage of patients with typhoid fever (n = 178) who had received a typhoid fever vaccine in the five years before illness, National Typhoid and Paratyphoid Fever Surveillance, by year, 2011–2015.1
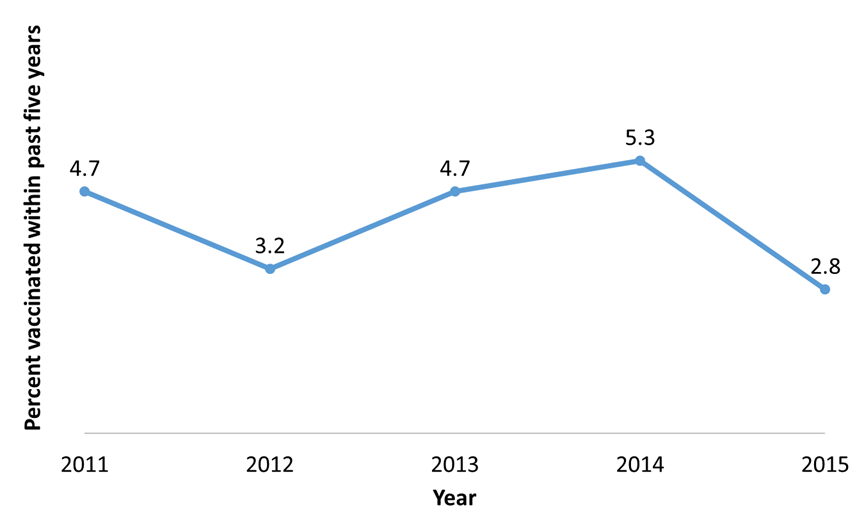
| Year | Percentage |
|---|---|
| 2011 | 4.7 |
| 2012 | 3.2 |
| 2013 | 4.7 |
| 2014 | 5.3 |
| 2015 | 2.8 |
1Excludes patients whose typhoid vaccination status was unknown or not reported.
National Notifiable Diseases Surveillance System
The National Notifiable Diseases Surveillance System (NNDSS) collects and compiles reports of nationally notifiable infectious diseases, including typhoid fever. The figure below presents a comparison of typhoid fever reports submitted to NTPFS and NNDSS in the past five years. Paratyphoid fever is excluded from this comparison, because it was nationally notifiable but reported under the case definition for salmonellosis during 2015.
Figure 4. Number of cases of typhoid fever reported to the National Notifiable Diseases Surveillance System (NNDSS) and to the National Typhoid and Paratyphoid Fever Surveillance (NTPFS), by year, 2011–2015
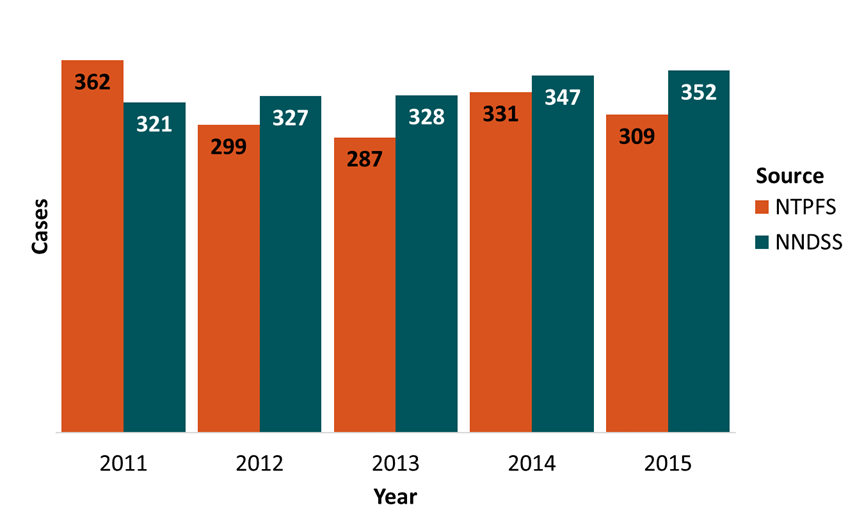
| Year | NTPFS | NNDSS |
|---|---|---|
| 2011 | 362 | 321 |
| 2012 | 299 | 327 |
| 2013 | 287 | 328 |
| 2014 | 331 | 347 |
| 2015 | 309 | 352 |
Antimicrobial Resistance
The National Antimicrobial Resistance Monitoring System (NARMS) monitors antimicrobial resistance among enteric bacteria (including Salmonella serotypes Typhi and Paratyphi A, Paratyphi B [tartrate negative], and Paratyphi C) from humans. Among Typhi, decreased susceptibility to ciprofloxacin (DSC) (MIC ≥0.12 µg/mL) has been associated with fluoroquinolone treatment failure. Multidrug-resistant (MDR) is defined as resistance to ampicillin, chloramphenicol, and trimethoprim-sulfamethoxazole.
The most recently published NARMS Annual Report is from 2015.
According to the NARMS 2015 Human Isolates Surveillance Report:
For Salmonella serotype Typhi isolates:
- 66% had DSC
- 8.9% were MDR
- 11.6% were resistant to three or more classes of antibiotics
- No isolates were resistant to ceftriaxone
For Salmonella serotype Paratyphi A isolates:
- 89% had DSC
- No isolates were resistant to ceftriaxone
Outbreaks
The Foodborne Disease Outbreak Surveillance System (FDOSS) collects reports of foodborne disease outbreaks from local, state, tribal, and territorial public health agencies. The most recently published annual report is from 2015 [PDF – 24 pages].
The Waterborne Disease and Outbreak Surveillance System (WBDOSS) collects reports of waterborne disease outbreaks associated with drinking water and recreational water from local, state, tribal, and territorial public health agencies.
There were two outbreaks of typhoid fever and one outbreak of paratyphoid fever reported in 2015. All were confined to single states:
- An outbreak of typhoid fever in Oklahoma resulted in 25 illnesses.
- An outbreak of typhoid fever in Colorado resulted in 3 illnesses. It was linked to a restaurant worker who was a chronic carrier.
- An outbreak of paratyphoid fever in Michigan caused by Paratyphi A resulted in 3 illnesses.
- National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS): Human Isolates Surveillance Report for 2014 (Final Report). Atlanta, Georgia: U.S. Department of Health and Human Services, CDC, 2016.
- Date KA, Newton AE, Medalla F, Blackstock A, Richardson L, McCullough A, et al. Changing patterns in enteric fever incidence and increasing antibiotic resistance of enteric fever isolates in the United States, 2008–2012. Clin Infect Dis. 2016 Aug 1;63(3):322–9.
Centers for Disease Control and Prevention (CDC). National Typhoid and Paratyphoid Fever Surveillance Annual Summary, 2015. Atlanta, Georgia: US Department of Health and Human Services, CDC, 2018.