Recommendations for Fully Vaccinated People
COVID-19 Homepage
FAQ: COVID-19 Data and Surveillance
Frequently Asked Questions
- Below are answers to commonly asked questions about CDC’s COVID-19 data and surveillance across the country, as well as collecting and sharing data with the public.
- COVID Data Tracker serves as CDC’s home for COVID-19 data.
National COVID-19 Case Surveillance
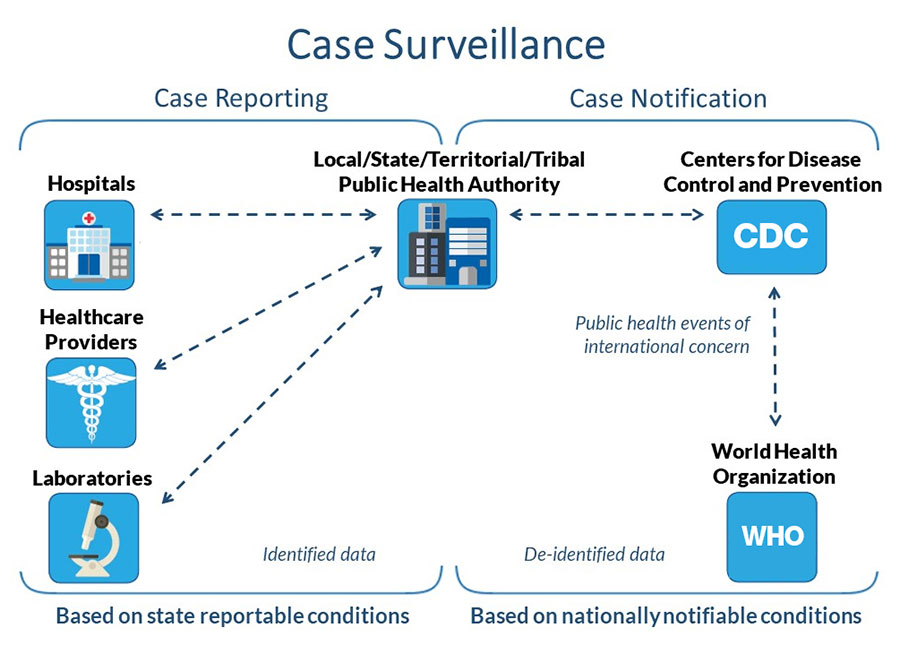
Public health departments routinely collect information on people with certain infections. This process, known as case surveillance, can help officials understand where, when, and in which populations an illness is transmitted. This supports action to control outbreaks and prevent the spread of disease. Nationally, more than 120 diseases and conditions are tracked. The information collected is referred to as case data. These efforts are part of a wider ongoing process called public health surveillance. The goal is to protect Americans from infectious diseases and other health threats.
Case surveillance is especially important for new diseases, such as COVID-19. The information collected helps identify similarities and differences among cases. Information commonly collected includes:
- Demographic information (age, race, ethnicity, etc.)
- Clinical factors, such as symptoms
- Epidemiologic characteristics (where, when, and in which populations an illness is transmitted)
- Exposure and contact history (how an illness is spreading)
- Course of clinical illness and care received
State, local, and territorial health departments transmit case data to CDC through the National Notifiable Diseases Surveillance System (NNDSS). This process is voluntary. To protect individual privacy, all information transmitted to CDC is de-identified. This means it does not contain any personal identifiers, such as names or home addresses, and cannot be linked to an individual. Learn more on How We Conduct Case Surveillance.
Case information is gathered through a data supply chain, which is a process for reporting, collecting, and analyzing disease data. Steps include the following:
- Hospitals, healthcare providers, and laboratories transfer data for case reporting to state, local, and territorial public health departments as required under state disease reporting laws.
- State, local, and territorial health departments move data for case notification to CDC through NNDSS. This step is voluntary, and all data is de-identified before transmission to CDC.
- CDC reports national COVID-19 case surveillance data to the World Health Organization, as required under International Health Regulations. CDC also publishes COVID-19 national case surveillance data for public use at data.cdc.gov.
CDC uses two data sources to obtain information on COVID-19 cases. The first is aggregate count data, which offer high-level information about case and death totals. Data are gathered through a robust process with the following steps:
- Aggregate county-level counts are obtained indirectly, via automated overnight web collection, or directly, via a data submission process.
- A CDC data team reviews counts for congruency prior to integration.
- CDC compiles these data and posts finalized county-level, jurisdictional, and national aggregate counts on the COVID Data Tracker
This process is collaborative, with CDC and jurisdictions working together to ensure the accuracy of COVID-19 case and death numbers. Aggregate counts provide the most up-to-date numbers on cases and deaths based on date of report. CDC may retrospectively update counts as jurisdictions provide updated information.
The second data source involves line-level (patient-level) data. This provides specific information for each case. CDC receives line-level data primarily from state health departments. Information is de-identified and does not include names or home addresses. CDC makes line-level data available through patient-level data sets. The COVID Data Tracker also features some of this information.
Line-level data includes:
- Patient demographics such as age, race, and ethnicity
- Signs and symptoms of illness
- Underlying health conditions
- Characteristics of hospitalizations, such as ventilator use
- Clinical outcomes
- Exposures
Because it can be time-consuming for jurisdictions to collect the additional information, line-level data reporting can take more time than aggregate count reporting. CDC receives this information for most, but not all, cases.
CDC uses multiple public health surveillance systems to monitor COVID-19. This includes influenza and viral respiratory disease surveillance, syndromic surveillance, lab reporting, health care systems reporting, research platforms, vital statistics, and new surveillance systems designed to answer specific questions. This combined information offers an updated picture of the spread and impact of COVID-19.
- Why is CDC changing the COVID-19 Line Level and Aggregate Case and Death Data reporting cadence from daily to weekly?
- To allow for additional reporting flexibility, reduce the reporting burden on states and jurisdictions, and maximize surveillance resources, CDC is moving to a weekly reporting cadence for line level and aggregate case and death data.
- When will this change from daily to weekly COVID-19 surveillance cadence go into effect?
- On October 20, 2022, CDC will report updates to COVID-19 aggregate case and death data and line level data on a weekly, rather than daily, cadence. Data processing cutoffs for jurisdictions will be every Wednesday at 10AM ET for line level case and death data, and Wednesday at 5PM ET for direct submission of aggregate case and death data.
The COVID-19 pandemic has strained the public health data supply chain. In many states, this has challenged hospitals, healthcare providers, and laboratories in reporting complete demographic information, such as race and ethnicity. The volume of cases has also made it challenging for state, local, and territorial health departments to conduct thorough investigations. As a result, some COVID-19 case notifications do not have complete information, even as health departments continue to make improvements through methods such as automated data flows.
Missing data can affect interpretation of factors that might put people at higher risk for severe disease. Analyses of incomplete data elements are likely an underestimate of the true occurrence.
Case surveillance provides information on the characteristics of a disease within a population. Cases are identified using a standard case definition and are typically confirmed through laboratory testing. CDC uses national case surveillance to:
- Track the spread of COVID-19 to identify areas of concern and inform state decision-makers.
- Help state and local public health departments better control COVID-19 by evaluating trends in case demographics, exposures, and outcomes to identify groups most at risk. Examples would include healthcare workers, racial and ethnic minority groups, older adults, and people with certain underlying health conditions.
- Analyze exposure information and health outcomes among COVID-19 patients. This can help in developing guidance for the public, at-risk groups, and healthcare providers.
National case surveillance data are constantly changing. As new information is gathered about previously reported cases, health departments provide updated data to CDC. As a result, surveillance data and trends from a previously reported time window might keep changing.
Another key challenge is that some people infected with the virus that causes COVID-19 have mild or no symptoms. If testing and health care services are not needed, those people are less likely to be reported as cases. Similarly, people who have had severe outcomes – such as hospitalization, intensive care unit (ICU) admission, and death – are more likely to be reported as cases. These challenges can limit analysis and interpretation of the data.
Yes, a CDC data team communicates directly with jurisdictions to account for instances of delayed or late reporting. Updates will be made to aggregate COVID-19 data at the jurisdictional-level and/or county-level when available.
CDC continues to work with state, local, and territorial health departments. Goals include accelerating reporting of national case surveillance data, improving data quality, and gathering complete information about all COVID-19 cases.
Another improvement initiative involves continuing to modernize disease surveillance through electronic case reporting. This process allows for automated, real-time exchange of case report information. Data flows seamlessly from a healthcare provider’s electronic health record (EHR) to a public health agency. This supports timely review and action for COVID-19 cases. Electronic case reporting is a joint effort involving healthcare providers, EHR vendors, and state, local, and territorial health departments.
CDC offers many resources, including the following:
CDC COVID Data Tracker – This serves as CDC’s home for COVID-19 data. Case and death counts reported to CDC since January 21, 2020 are available here. COVID Data Tracker is updated frequently. Timing depends on the availability of data provided by jurisdictions. Information about the frequency of updates is available on each data page. Topics covered on COVID Data Tracker include:
- COVID-19 in Your Community
- Vaccinations
- Cases, Deaths, and Testing
- Health Equity Data
- Demographic Trends
- Health Care Settings
- Genomic Surveillance
- Seroprevalence
- People at Increased Risk
COVID Data Tracker Weekly Review
- Key updates for the week (trends in cases, deaths, variants, laboratory testing, hospitalizations, and vaccinations)
- Interpretive summaries for trends in key COVID-19 data
Hospitalization Surveillance Network (COVID-NET)
- National hospitalization rates for COVID-19
- Characteristics of people hospitalized with COVID-19 in the U.S.
COVID-19 Data from the National Center for Health Statistics (NCHS)
- Provisional death counts based on death certificate data from the National Vital Statistic System.
- Data on mental health and access to health care from the NCHS partnership with the U.S. Census Bureau on the Household Pulse Survey. (Includes indicators of anxiety and depression based on reported frequency of symptoms during the last seven days.)
- Maternal and infant characteristics among women with confirmed or presumed COVID-19 during pregnancy.
Current Hospital Capacity Estimates
- Estimated percentage of inpatient beds occupied by all patients, by state.
- Estimated percentage of inpatient beds occupied by COVID-19 patients, by state.
- Estimated percentage of ICU beds occupied by all patients, by state.
Other resources:
- CDC’s National Notifiable Diseases Surveillance System
- Electronic Case Reporting for COVID-19
- CSTE Case Definition for COVID-19
- International Health Regulations (2005)
- Public Health Surveillance in the United States: Evolution and Challenges, July 2012
- Modernizing Centers for Disease Control and Prevention Informatics Using Surveillance Data Platform Shared ServicesExternal, March-April 2018
- CDC’s Vision for Public Health Surveillance in the 21st CenturyPdf, July 2012
- Centers for Disease Control and Prevention (CDC). Introduction to Public Health. In: Public Health 101 Series. Atlanta, GA: U.S. Department of Health and Human Services, CDC; 2014.
- CDC MMWR Novel Coronavirus Reports
Understanding COVID-19 Case Data
A COVID-19 case is an individual who has been determined to have COVID-19 using a set of criteria known as a case definition. Cases can be classified as suspect, probable, or confirmed. CDC counts include probable and confirmed cases and deaths. Suspect cases and deaths are excluded.
The case classifications for COVID-19 are described in an revised COVID-19 position statement and case definition issued by the Council of State and Territorial Epidemiologists. A probable case or death is defined as any one of the following:
- Meets clinical criteria AND epidemiologic linkage with no confirmatory laboratory testing performed for SARS-CoV-2
- Meets presumptive laboratory evidence
- Meets vital records criteria with no confirmatory laboratory evidence for SARS-CoV-2
Any cases and deaths classified as probable are included in CDC case counts. The same applies to any cases and deaths classified as confirmed.
Antigen tests administered by a Clinical Laboratory Improvement Amendments (CLIA)-certified provider, when positive, meet the criteria for identifying a person as a probable case according to the current Council of State and Territorial Epidemiologists guidance (21-ID-01). However, a positive antigen test does not meet the criteria for a confirmed case, which must be identified based on a positive nucleic acid amplification test (NAAT) performed in a CLIA-certified laboratory.
Reporting of antigen results by CLIA-certified providers to local and state public health agencies is required under the Coronavirus Aid, Relief, and Economic Security (CARES) Act (P.L. 116-136, § 18115) and by most state and local health departments. If a jurisdiction uses antigen results to identify probable cases and reports those cases to CDC, these numbers are included in the jurisdictional case totals. This information is accessible on the CDC COVID Data Tracker.
Cases based solely on a positive self-test are not included in national or state COVID-19 case counts. This is because self-tests are not administered by a CLIA-certified provider and do not meet the criteria for a confirmed or probable case.
[Additional information on case definitions can be found here: Coronavirus Disease 2019 (COVID-19) 2021 Case Definition | CDC and here: CDC COVID Data Tracker]
CDC and state and local public health agencies rely on multiple data types to understand disease burden. While increases in self-testing have the potential to impact case data and laboratory data, both data sources will continue to track trends in illness and community transmission. Other types of data also help provide insight into aspects of disease burden such as the demands on healthcare systems, highly impacted or disproportionately impacted populations, and disease severity indicators.
The virus that causes COVID-19 spreads easily and sustainably between people. The more closely people interact and the longer that interaction, the higher the risk of COVID-19 spread. Differences in community characteristics and changes in preventive behavior can result in increases or decreases of cases. Changes in the virus (mutations) can also lead to changes in the number of cases.
The count on the Cases, Deaths, and Testing page includes deaths reported by state, local, and territorial health departments. Reporting frequency might vary by jurisdiction. This reflects the most up-to-date information received by CDC based on preliminary reporting.
In contrast, provisional COVID-19 death counts from the National Center for Health Statistics (NCHS) are updated with information from death certificates. This offers the most accurate death counts, but there is a reporting lag time of one to two weeks on average. Death counts are continually updated as new death certificate data are received. For these reasons, provisional COVID-19 death counts might differ from those on other published sources.
The mortality rate is the number of people who died due to COVID-19 divided by the total number of people in the population. Since this is an ongoing outbreak, the mortality rate can change daily. CDC reports COVID-19 deaths in the COVID Data Tracker.
Organizations use various methods to collect and report data, which can account for some of these differences. CDC checks overall case numbers through a confirmation process with each jurisdiction. Differences between data displayed by reporting jurisdictions and CDC’s website might occur due to the timing of reporting and timing of website updates.
Limitations of using case surveillance data to understand the epidemiology (who, what, where, when, how) of COVID-19 include the following:
- Case surveillance data do not represent the true burden of COVID-19 in the United States. Many people infected, even if symptomatic, do not seek medical care or get tested. In these cases, data cannot be extracted from medical records. Data can also be limited if people are unavailable or unwilling to provide information.
- Most of the case reports captured by health departments are based on laboratory reports that might contain limited patient information. Because of the volume of cases, most health departments are unable to obtain additional information on every case. As a result, many case reports are missing data on patient demographics, symptoms, underlying health conditions, characteristics of hospitalizations such as ventilator use, and other factors such as travel history. Because of missing data, analyses of these data elements are likely an underestimate of the true occurrence.
- It is difficult to capture asymptomatic cases through case surveillance. People who are asymptomatic are unlikely to seek testing unless they are identified through active screening such as contact tracing. In general, investigation of symptomatic people is prioritized.
When disease volume is high and a limited number of data elements are captured on each reported case, case surveillance data can be used to assess the following:
- Population burden
- Spread
- Increases and decreases in cases in association with mitigation strategies
- Selected demographics such as age, sex, race, ethnicity, and geography
Clinical details and other characteristics about people with COVID-19 can be better assessed through special studies. CDC conducts these special epidemiologic studies to better understand risk factors, such as underlying conditions that might put people at increased risk for serious infection. CDC also conducts special studies using hospitalization and treatment data to better understand the clinical course of COVID-19 illness.
New COVID-19 cases and deaths are recorded based on data collected and reported by state, local, and territorial health departments. This information can be affected by local testing practices, laboratory capacity, and medical resources. Comparing the COVID-19 situation among jurisdictions should not be based on these rates alone.
When studying the COVID-19 situation in these jurisdictions, the rate of new cases should be assessed alongside other data. This could include the number of tests performed, the proportion of tests that are positive for SARS-CoV-2, testing policies, excess deaths, and hospital and ICU admission rates.
In addition, jurisdictions might inconsistently report demographic data, including race and ethnicity, for COVID-19 cases. Because racial and ethnic composition varies, comparisons of COVID-19 case information should consider the population of each geographic area. For these reasons, CDC’s case data might not be generalizable to the entire U.S. population.
Determining COVID-19 Community Levels
COVID-19 Community Levels are a tool to help communities decide what prevention steps to take based on the latest data. Levels can be low, medium, or high and are determined by looking at hospital beds being used, hospital admissions, and the total number of new COVID-19 cases in an area.
At each of the three levels—low, medium, and high—CDC recommends specific personal and community prevention measures. CDC’s COVID-19 Community Levels recommend certain prevention measures to avoid strain on the healthcare system and protect those at increased risk of severe illness.
The COVID-19 Community Levels assess data related to the proportion of hospital capacity devoted to caring for COVID-19 patients, the number of new patients with COVID-19 admitted to the hospital in the last 7 days, and the number of new COVID-19 cases in the county in the last 7 days. These data tell us how much the virus is spreading in an area, how many people in the area are getting sick to the extent that requires medical care, and if the area’s healthcare system has enough resources to provide care for all patients, whether or not they have COVID-19.
CDC evaluated how these metrics perform individually and how well COVID-19 Community Levels predict what will happen in communities up to 6 weeks later. These analyses showed that the COVID-19 Community Levels provide a sizeable improvement over community transmission levels in identifying regions that will experience severe outcomes—including ICU admissions and deaths—in the weeks ahead. Thresholds were selected based on these analyses and were validated through discussion with and input from local and state health officials and experts from hospitals and healthcare systems. These thresholds are designed to signal the need for additional precautions before a worsening trend leads to a crisis. Read more about the process: Science Brief: Indicators for Monitoring COVID-19 Community Levels and Making Public Health Recommendations.
Reporting on a weekly basis improves stabilization of the COVID-19 Community Levels, allowing counties to make informed decisions and take appropriate actions. This is especially important for counties in health service areasPdf with smaller populations or fewer hospital facilities, where slight differences in numbers can have a greater impact on the rates calculated in a given day.
Using health service areas (HSAs) makes it possible to calculate metrics of health care resource availability (i.e., new admissions of patients with confirmed COVID-19 and percent of staffed in-patient beds with confirmed COVID-19) at a substate geographic level, while accounting for counties that do not have hospitals. Each county within a given HSA will be attributed the metrics calculated for the entire HSA.
An HSA is defined by CDC’s National Center for Health Statistics as a geographic area containing at least one county that is self-contained with respect to the population’s provision of routine hospital care. Every county in the United States is assigned to an HSA, and each HSA must contain at least one hospital. Although the county is typically the smallest geographic unit for which national data are available, not all counties in the United States have hospitals. Additionally, analyses of hospital data at the local level are complicated by unequal distribution of hospitals within regions, as they are often clustered in large population centers and have service areas that overlap and extend across multiple communities. This can be particularly true for children’s or other specialty hospitals. The unequal distribution of hospitals can lead to a mismatch between places where people live and places where they receive care.
Therefore, use of HSAs in the calculation of local hospital metrics, including the COVID-19 Community Level indicators, allows for more accurate characterization of the relationship between health care utilization and health status at the local level.
For access to Health Service Area (HSA) mappings used in COVID-19 Community Level, please refer to the data table and CSV, under “U.S. COVID-19 Community Levels by County Map” on this CDC webpage: https://www.cdc.gov/coronavirus/2019-ncov/your-health/covid-by-county.html.
For more information about how the HSAs were determined, please refer to this resource:
- Makuc DM et al. Health service areas for the United States. National Center for Health Statistics. Vital Health Stat (2)112. 1991. https://www.cdc.gov/nchs/data/series/sr_02/sr02_112.pdfPdf.
There has been no change in the case rate metric between the previous Community Transmission approach and the new COVID-19 Community Levels. However, the case rate thresholds differ between the two. The table below compares the thresholds:
| New COVID-19 cases per 100,000 population (7-day total) |
Community Transmission* | COVID-19 Community Level** |
| <10 | Low | Low, Medium, or High |
| 10 – <50 | Moderate | Low, Medium, or High |
| 50 – <100 | Substantial | Low, Medium, or High |
| 100 – <200 | High | Low, Medium, or High |
| >200 | High | Medium or High |
-
- *Community Transmission is determined by the higher level associated with 7-day new COVID-19 cases per 100,000 population or 7-day percent positivity.
- **COVID-19 Community Level is determined by 7-day new COVID-19 cases per 100,000 population then the higher of 7-day new COVID-19 admissions per 100,000 population and 7-day average percent of staffed inpatient beds occupied by COVID-19 patients.
For COVID-19 Community Level, the thresholds are less than 200 cases per 100,000 population or 200 or more cases per 100,000 population, and then the higher of the hospital-based metrics. Any county with a 7-day new COVID-19 cases per 100,000 population that is 200 or more will fall into either the Medium or High category, based on the higher of the hospital-based metrics.
CDC uses county level data, which offer county-specific information about case and death totals. Since the start of the COVID-19 pandemic, data have been gathered through a robust process with the following steps:
- Aggregate county-level counts are obtained indirectly, via automated overnight web collection, or directly, via a data submission process.
- A CDC data team reviews counts for congruency prior to integration.
- CDC compiles these data and posts finalized county-level, jurisdictional, and national aggregate counts on the COVID Data Tracker
This process is collaborative, with CDC and jurisdictions working together to ensure the accuracy of COVID-19 case and death numbers. County counts provide the most up-to-date numbers on cases and deaths by report date. CDC may retrospectively update counts to correct data quality issues.
For more information on COVID-19 case data and surveillance, please visit FAQ: COVID-19 Data and Surveillance | CDC.
There has been no change in the case rate metric—a confirmed case must be identified based on a positive nucleic acid amplification test (NAAT) performed in a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory. A confirmed case is based on Council of State and Territorial Epidemiologists (CSTE) Interim-20-ID-02 guidancePdfExternal. See the section titled “Laboratory Criteria” for the specific laboratory criteria for a confirmed case.
Additional information on case definitions can be found at Coronavirus Disease 2019 (COVID-19) 2021 Case Definition and CDC COVID Data Tracker. Additional information on Clinical Laboratory Improvement Amendments can be found at Clinical Laboratory Improvement Amendments (CLIA).
Some discrepancies may be due to the differences in date ranges that are used for the case rates and hospital metrics, the time of day the analysis is run, rounding methods, or missing data.
- Date ranges used for individual metrics:
- The 7-day new cases per 100,000 population are calculated with a 1-day lag. For example, if the COVID-19 Community Level is calculated on day D, weekly cases are totaled for days [D-7, D-1] – e.g., for values calculated on Feb 10, the week would be Feb 3-9.
- The new admissions per 100,000 population and percent of staffed inpatient beds occupied by COVID-19 patients are calculated with a 2-day lag. For example, if the COVID-19 Community Level is calculated on day D, weekly admissions are totaled for days [D-8, D-2] – e.g., for values calculated on Feb 10, the week would be Feb 2-8. Similarly, occupancy is calculated for days [D-8, D-2] – e.g., for values calculated on Feb 10, the week would be Feb 2-8.
- Time of day running the analysis:
- Case rates are calculated after the datasets have been updated, which is usually done by noon (12pm ET).
- Admission rates and bed occupancy are calculated after the datasets have been updated, which is usually done around 9am ET.
- Rounding:
- To determine whether a case rate is above or below 200 cases per 100,000 population, round to a single decimal point. A community is defined to have greater than or equal to 200 cases per 100,000 population if it rounds to 200.0 or higher.
- Missing data:
- For case rates, if there are no data in the county for the given 7-day window, the category is considered low (under 200 case rate).
- For admission rates and percent of staffed inpatient beds occupied by COVID-19 patients, if there are no data in the county for the given 7-day window, the category is “N/A”. If there are no data in the county for either of these metrics, the COVID-19 Community Level is “N/A.”
- To calculate the 7-day average percent of staffed inpatient beds occupied by COVID-19 patients, the average of valid values within the 7-day period is taken (e.g., if only 3 valid values, the average of those 3 is taken).
- 7-day averages are used rather than daily values because there are weekly cycles in both utilization patterns and reporting patterns that might cause values to be noticeably lower/higher on certain days of the week otherwise.
Hospital data are sourced from the U.S. Department of Health and Human Services (HHS) Unified Hospital Data Surveillance System (UHDSS). All hospitals in the United States report to UHDSS in accordance with the COVID-19 guidance for hospital reportingPdfExternal.
The following resource provides HHS COVID-19 Guidance for Hospital Reporting and FAQs For Hospitals, Hospital Laboratory, and Acute Care Facility Data Reporting: https://www.hhs.gov/sites/default/files/covid-19-faqs-hospitals-hospital-laboratory-acute-care-facility-data-reporting.pdfPdfExternal
Hospitals report data directly to the U.S. Department of Health and Human Services (HHS) or via a state submission for collection in the HHS Unified Hospital Data Surveillance System (UHDSS); detailed reporting guidance and full list of data elements collected by UHDSS can be found here: https://www.hhs.gov/sites/default/files/covid-19-faqs-hospitals-hospital-laboratory-acute-care-facility-data-reporting.pdfPdfExternal).
More than 6,000 hospitals across the United States report data to UHDSS daily. While CDC reviews these data for errors and corrects those found, some reporting errors may still exist. To minimize errors and inconsistencies, CDC removes outliers before calculating the metrics. CDC and partners, such as HHS and Office of the Assistant Secretary for Preparedness and Response (ASPR) work with reporting hospitals to correct these errors and update the data in UHDSS at a later date.
Many hospital subtypes, including acute care and critical access care hospitals, as well as Veterans Administration, Defense Health Agency, and Indian Health Service hospitals, are included in the metric calculations. Psychiatric, rehabilitation, and religious non-medical hospital types are excluded. For a full list of hospital subtype descriptions, please visit the detailed reporting guidance (https://www.hhs.gov/sites/default/files/covid-19-faqs-hospitals-hospital-laboratory-acute-care-facility-data-reporting.pdfPdfExternal).
For hospitals with the same Centers for Medicare and Medicaid Services (CMS) Certification Number (CCN), data are aggregated for display. CMS assigns CCNs to hospitals which are mapped to counties, based on the CMS Provider of Services files (https://www.cms.gov/Research-Statistics-Data-and-Systems/Downloadable-Public-Use-Files/Provider-of-ServicesExternal). Approximately 3% of CCNs contain multiple-component hospitals that map to different counties; and 1.8% contain multiple-component hospitals that map to different Health Service Areas.
Other relevant information, as well as additional detailed calculations, can be viewed on
CDC COVID Data Tracker, under the “Footnotes” section.
All hospitals in the United States registered with Centers for Medicare & Medicaid Services (CMS) are required to report to UHDSS in accordance with the COVID-19 guidance for hospital reportingPdfExternal. Hospital reporting rates are typically over 95%. A public visualization of the overall percentage of hospitals reportingExternal one or more elements for the previous week is updated weekly.
All hospitals in the United States registered with CMS are required to report to UHDSS in accordance with the COVID-19 guidance for hospital reportingPdfExternal. All hospitals should report at the individual hospital level, even if hospitals share a CMS Certification Number (CCN).
There are several mechanisms for reporting hospital data into UHDSS. These include:
– Hospital → UHDSS
– Hospital → hospital system enterprise level (centralized reporting) -> UHDSS
– Hospital → health IT vendor or other third-party situational awareness provider -> UHDSS
– Hospital → “certified” state-> UHDSS
*Note: A list of certified states is available on healthdata.govExternal; a state being certified does not mean that some or all of the hospitals report through this mechanism.
Differences can often be attributed to differences in questions and/or definitions between federal and state hospital data collections. Differences may also be related to hospital entry information, depending on whether a hospital reports directly to the state, and/or whether a state reports on behalf of a hospital.
CDC looks at the combination of three metrics — new COVID-19 admissions per 100,000 population in the past 7 days, the percent of staffed inpatient beds occupied by COVID-19 patients, and total new COVID-19 cases per 100,000 population in the past 7 days — to determine the COVID-19 Community Level. Data for these indicators are publicly available:
Hospital
- COVID-19 Reported Patient Impact and Hospital Capacity by StateExternal
- COVID-19 Reported Patient Impact and Hospital Capacity by FacilityExternal
- COVID-19 Reported Patient Impact and Hospital Capacity by State TimeseriesExternal
Aggregate County Surveillance:
Learn more about the data and scientific rationale at COVID-19 Community Levels.
Surveillance Reports
Yes. On February 12, 2021, we posted the first COVID Data Tracker Weekly Review, which is shared every Friday. This newsletter highlights key data from CDC’s COVID Data Tracker. It summarizes important trends in the pandemic and centralizes CDC data and reporting.
The COVID Data Tracker Weekly Review replaced the COVIDView report, which was produced weekly from April 3, 2020, through February 5, 2021. An archive of COVIDView reports is maintained on the CDC website.
COVID-19 surveillance data are also used to produce publications, including CDC’s Morbidity and Mortality Weekly Report (MMWR), and to inform guidance documents to protect people from COVID-19 in a variety of settings.
CDC COVID Data Tracker
CDC COVID Data Tracker is CDC’s home for COVID-19 data. It provides surveillance data from across the response, including hospitalizations, vaccinations, demographic information, and daily and cumulative case and death counts reported to CDC since January 21, 2020. COVID Data Tracker is updated frequently. Timing depends on the availability of data provided by jurisdictions. Information about the frequency of updates is available on each data page. Topics covered on COVID Data Tracker include:
- COVID-19 in Your Community
- Vaccinations
- Cases, Deaths, and Testing
- Health Equity Data
- Demographic Trends
- Health Care Settings
- Genomic Surveillance
- Testing and Seroprevalence
- People at Increased Risk
COVID Data Tracker is updated frequently. Timing depends on the availability of data provided by jurisdictions. Information about the frequency of updates is available on each data page.
Yes, there are multiple datasets that can be downloaded directly from COVID Data Tracker. To download data from COVID Data Tracker, navigate to the data table in the tab you are viewing and click on the download icon.
Download-solid
To download case and death data over time, including historical data, visit the U.S. and State Trends page and click the Download Chart button. Alternatively, the data can be downloaded at data.cdc.gov.
To download case and death data over time, including historical data, visit the U.S. and State Trends page and click the Download Chart button. Alternatively, the data can be downloaded at data.cdc.gov.
You can download line-level data, including patient sex, age group, hospitalization status, and race/ethnicity, county, and state of residence (where available) from three available COVID-19 case surveillance datasets.
You can conduct your own analyses using the available datasets to determine the number and selected characteristics of lab-confirmed cases shared with CDC by jurisdictions through a specific date. You can download de-identified CDC case surveillance data, which includes fields for date of first positive specimen collection, case status (lab-confirmed vs. probable), and others. See the next section titled: “CDC Publicly Available Datasets.”
CDC Publicly Available Datasets
Sharing timely and accurate COVID-19 data with the public is a core activity of CDC’s COVID-19 Emergency Response as well as a key priority of CDC’s Data Modernization Initiative, and the administration’s Executive Order on Ensuring a Data-Driven Response to COVID-19 and Future High-Consequence Public Health Threats. Publicly available datasets are critical for several reasons: open government and transparency, promotion of research, and efficiency (i.e., providing the public, media, and others access to the same data with consistency and supporting information).
CDC has three COVID-19 case surveillance datasets:
- COVID-19 Case Surveillance Public Use Data with Geography: Public use, patient-level dataset with clinical and symptom data, demographics, and state and county of residence. This dataset contains 19 data elements.
- COVID-19 Case Surveillance Public Use Data: Public use, patient-level dataset with demographic and clinical information, including symptoms. No geographic data is available. This dataset contains 12 data elements.
- COVID-19 Case Surveillance Restricted Access Detailed Data: Restricted access, patient-level dataset with demographic and clinical data, including symptoms. Geographic data (state and county of residence) is available. Access requires a registration process and a data-use agreement. This dataset contains 33 data elements.
To reduce the risk that these datasets could be used to reidentify persons, CDC designed each dataset accounting for privacy and confidentiality, and conducts ongoing privacy assessments using standard methods and systematically verifies the data prior to release. Strict privacy protections, including data suppression, were applied to all three datasets. See the information included with each dataset for more information.
Although the CDC COVID Data Tracker and health department websites also report COVID-19 case surveillance data, data might not match the CDC public use datasets due to differences in timing of the creation of the datasets and differences in the timing of reporting and case notification. The three COVID-19 case surveillance datasets are updated every two weeks, and there is a reporting lag. The CDC COVID-19 Data Tracker is updated frequently. Timing depends on the availability of data provided by jurisdictions. Information about the frequency of updates is available on each data page. When there are differences between numbers of cases reported, data reported by health departments should be considered the most up to date for the state or territory.