Recommendations for Fully Vaccinated People
COVID-19 Homepage
Science Brief: COVID-19 Vaccines and Vaccination
COVID-19 Science Briefs provide a summary of the scientific evidence used to inform specific CDC guidance and recommendations. The Science Briefs reflect the scientific evidence, and CDC’s understanding of it, on a specific topic at the time of the Brief’s publication. Though CDC seeks to update Science Briefs when and as appropriate, given ongoing changes in scientific evidence an individual Science Brief might not reflect CDC’s current understanding of that topic. As scientific evidence and available information on COVID-19 change, Science Briefs will be systematically archived as historic reference materials.
Page First Published March 8, 2021 | View Page Updates
Key Points
- All COVID-19 vaccines currently approved or authorized in the United States (Pfizer-BioNTech/Comirnaty, Moderna, and Janssen [Johnson & Johnson]) are effective against COVID-19, including against severe disease, hospitalization, and death.
- Available evidence suggests the currently approved or authorized COVID-19 vaccines are highly effective against hospitalization and death for a variety of strains, including Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), and Delta (B.1.617.2); data suggest lower effectiveness against confirmed infection and symptomatic disease caused by the Beta, Gamma, and Delta variants compared with the ancestral strain and Alpha variant. Ongoing monitoring of vaccine effectiveness against variants is needed.
- Limited available data suggest lower vaccine effectiveness against COVID-19 illness and hospitalization among immunocompromised people. In addition, numerous studies have shown reduced immunologic response to COVID-19 vaccination among people with various immunocompromising conditions.
- The risk for SARS-CoV-2 infection in fully vaccinated people cannot be completely eliminated as long as there is continued community transmission of the virus. Early data suggest infections in fully vaccinated persons are more commonly observed with the Delta variant than with other SARS-CoV-2 variants. However, data show fully vaccinated persons are less likely than unvaccinated persons to acquire SARS-CoV-2, and infections with the Delta variant in fully vaccinated persons are associated with less severe clinical outcomes. Infections with the Delta variant in vaccinated persons potentially have reduced transmissibility than infections in unvaccinated persons, although additional studies are needed.
Background
COVID-19 vaccination is a critical prevention measure to help end the COVID-19 pandemic. COVID-19 vaccines are now widely available in the United States, and CDC recommends all people 12 years and older be vaccinated against COVID-19.
On August 23, 2021, the U.S. Food and Drug Administration (FDA) approved an mRNA vaccine (Pfizer-BioNTech/Comirnaty) as a 2-dose series for prevention of symptomatic COVID-19 in persons aged ≥16 years. This vaccine is also authorized under an Emergency Use Authorization (EUA) to be administered to prevent COVID-19 in persons aged 12-15 years. A second mRNA vaccine (Moderna), as well as a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector vaccine (Janssen vaccine [Johnson & Johnson]) are authorized under an EUA for use in persons aged ≥18 years. Both mRNA vaccines are also authorized for administration of an additional dose to certain immunocompromised persons.
People are considered fully vaccinated if they are ≥2 weeks following receipt of the second dose in a 2-dose series (mRNA vaccines), or ≥2 weeks following receipt of a single-dose vaccine (Janssen vaccine).*
Public health recommendations for people fully vaccinated with FDA-approved or FDA-authorized COVID-19 vaccines consider evidence of vaccine effectiveness against symptomatic COVID-19 with and without severe outcomes, as well as vaccine impact on SARS-CoV-2 transmission. Other individual and societal factors are also important when evaluating the benefits and potential harms of additional prevention measures (e.g., masking, physical distancing) among vaccinated individuals. The Advisory Committee on Immunization Practices and CDC routinely consider individual health benefits and risks along with factors such as population values, acceptability, and feasibility of implementation when making vaccine recommendations.(1) These factors were also considered when developing CDC’s interim public health recommendations for fully vaccinated people.
In this scientific brief, we summarize evidence available through August 24, 2021, for the currently approved or authorized COVID-19 vaccines (administered according to the recommended schedules) and additional considerations used to inform public health recommendations for fully vaccinated people, including:
- Vaccine efficacy and effectiveness against SARS-CoV-2 infection in the general population as well as among immunocompromised persons
- Vaccine effectiveness of heterologous (mixed) vaccination series
- Vaccine performance (i.e., immunogenicity and effectiveness) against emerging SARS-CoV-2 variant viruses, with a particular focus on the Delta (B.1.617.2) variant
Current evidence indicates that fully vaccinated people without immunocompromising conditions are able to engage in most activities with low risk of acquiring or transmitting SARS-CoV-2, with additional prevention measures (e.g. masking) where transmission is substantial or high.
Emerging SARS-CoV-2 viral variants
As of August 28, 2021, the Delta variant of concern (B.1.617.2) is the predominant variant in the United States, with 99% of sequenced specimens being identified as Delta; current data on variant prevalence can be found on CDC’s website. The Delta variant, first detected in India, has been shown to have increased transmissibility, potential reduction in neutralization by some monoclonal antibody treatments, and reduction in neutralization by post-vaccination sera.(2)
Other variants that are either no longer detected or are circulating at very low levels in the United States include: Alpha (B.1.1.7), first detected in the United Kingdom; Beta (B.1.351), first detected in South Africa; Gamma (P.1), first detected in Japan/Brazil; Iota (B.1.526), first detected in the United States-New York; Eta (B.1.525), first detected in the United Kingdom/Nigeria; Kappa (B.1.617.1) and B.1.617.3, first detected in India. These variants have mutations that alter the receptor binding domain of the spike protein and have variable impact on vaccine effectiveness (notably the E484K/Q mutation in Beta, Gamma, Eta, Iota, Kappa, and B.1.617.3; the N501Y mutation occurring in Alpha, Beta, and Gamma; the E417T/N mutations in Beta and Gamma; and the L452R mutation in Delta, Kappa and B.1.617.3).(2) Vaccine performance against emerging SARS-CoV-2 variants is an important consideration when evaluating the need for prevention measures in vaccinated people and will require continued monitoring.
COVID-19 vaccine efficacy, effectiveness, and immunogenicity
Immunogenicity is the generation of effective protective immunity against a vaccine antigen as measured by laboratory tests. Vaccine efficacy refers to how well a vaccine performs in a carefully controlled clinical trial, and effectiveness describes its performance in real-world observational studies. Evidence demonstrates that the approved or authorized COVID-19 vaccines are both efficacious and effective against symptomatic, laboratory-confirmed COVID-19, including severe forms of the disease. In addition, as shown below, a growing body of evidence suggests that COVID-19 vaccines also reduce asymptomatic infection and transmission. Substantial reductions in SARS-CoV-2 infections (both symptomatic and asymptomatic) will reduce overall levels of disease, and therefore, SARS-CoV-2 virus transmission in the United States. Investigations are ongoing to further assess the risk of transmission from fully vaccinated persons with SARS-CoV-2 infections to other vaccinated and unvaccinated people. Early evidence suggests infections in fully vaccinated persons caused by the Delta variant of SARS-CoV-2 may be transmissible to others; however, SARS-CoV-2 transmission between unvaccinated persons is the primary cause of continued spread.
Animal challenge studies
Rhesus macaque challenge studies provided the first evidence of the potential protective effects of Pfizer-BioNTech, Moderna, and Janssen COVID-19 vaccines against SARS-CoV-2 infection, including both symptomatic and asymptomatic infection. Vaccinated macaques developed neutralizing antibodies that exceeded those in human convalescent sera and showed no or minimal signs of clinical disease after SARS-CoV-2 challenge.(3-5) In addition, COVID-19 vaccination prevented or limited viral replication in the upper and lower respiratory tracts, which may have implications for transmission of the virus among humans.(3-5)
Vaccine efficacy from human clinical trials
Clinical trials subsequently demonstrated the FDA-approved or authorized COVID-19 vaccines to be efficacious against laboratory-confirmed, symptomatic COVID-19 in adults, including severe forms of the disease, with evidence for protection against both symptomatic and asymptomatic SARS-CoV-2 infection (6-12) (Box). Trial data demonstrated 100% efficacy of the Pfizer-BioNTech vaccine against laboratory-confirmed, symptomatic COVID-19 in adolescents 12–15 years old; this estimate was based on small numbers of cases and prior to emergence of the Delta variant.(13)
Clinical trial data suggest that the Janssen COVID-19 vaccine may have reduced overall efficacy against disease caused by the Beta variant, compared to the other COVID-19 vaccines. Although sero-response rates were similar between U.S. clinical trial participants and those from Brazil and South Africa, vaccine efficacy against moderate to severe-critical COVID-19 after ≥14 days was 74% in the United States (where ~96% of infections were due to the ancestral strain with the D614G mutation), 66% in Brazil (where ~69% of infections were due to Zeta [P.2]), and 52% in South Africa (where ~95% of infections were due to Beta).(14) Notably, Janssen vaccine showed good efficacy against severe or critical disease (73%–82%) across all sites.
Box. Summary of vaccine efficacy estimates for approved or authorized COVID-19 vaccines
All approved or authorized COVID-19 vaccines demonstrated efficacy (range 65% to 95%) against symptomatic, laboratory-confirmed COVID-19 in adults ≥18 years.
- For each approved or authorized COVID-19 vaccine, efficacy was demonstrated across different populations, including elderly and younger adults, in people with and without underlying health conditions, and in people representing different races and ethnicities.
- The Pfizer-BioNTech COVID-19 vaccine also demonstrated high efficacy against symptomatic, laboratory-confirmed COVID-19 in adolescents aged 12-17 years.
All approved or authorized COVID-19 vaccines demonstrated high efficacy (≥89%) against COVID-19 severe enough to require hospitalization.
All approved or authorized COVID-19 vaccines demonstrated high efficacy against COVID-19-associated death.
- In the clinical trials, no participants who received a COVID-19 vaccine died from COVID-19; the Moderna and Janssen vaccine trials among adults ≥18 years each had COVID-19 deaths in the unvaccinated placebo arm.
Data from the clinical trials among adults ≥18 years old suggest COVID-19 vaccination protects against symptomatic infection and may also protect against asymptomatic infection.
- In the Moderna trial, among people who had received a first dose, the number of asymptomatic people who tested positive for SARS-CoV-2 at their second-dose appointment was approximately 67% lower among vaccines than among placebo recipients (0.1% [n=15] and 0.3% [n=39], respectively)
- Efficacy of Janssen COVID-19 vaccine against asymptomatic infection was 74% in a subset of trial participants.
No trials have compared efficacy between any of the approved or authorized vaccines in the same study population at the same time, making comparisons of efficacy difficult.
- All Phase 3 trials differed by calendar time and geography.
- Vaccines were tested in settings with different background COVID-19 incidence and circulating variants.
Vaccine effectiveness from real-world studies
Multiple studies from the United States and other countries have demonstrated that a two-dose COVID-19 mRNA vaccination series is effective against SARS-CoV-2 infection (including both symptomatic and asymptomatic infections) caused by ancestral and variant strains and sequelae including severe disease, hospitalization, and death. Early evidence for the Janssen vaccine also demonstrates effectiveness against COVID-19 in real-world conditions. There is now a substantial volume of scientific literature examining the effectiveness of COVID-19 vaccination against SARS-CoV-2 infection, symptomatic disease, and other clinical outcomes; detailed summaries of these studies are available in the International Vaccine Access Center’s VIEW-Hub resource library.
Several systematic reviews and meta-analyses of vaccine effectiveness have recently been published (15-17); meta-analyses indicate an average effectiveness of full vaccination against SARS-CoV-2 infection of 85%–95% shortly after completion of vaccination. (16, 17) However, many of the studies in these reviews were conducted prior to the emergence of the variants of concern. Studies in Israel, Europe, and the United Kingdom have demonstrated high real-world effectiveness (>85%) of two doses of Pfizer-BioNTech COVID-19 vaccine while the Alpha variant was prevalent.(18-26) Studies from Qatar have demonstrated high effectiveness against documented infection with Alpha and Beta ≥14 days after receiving the Pfizer-BioNTech vaccine (90% and 75%, respectively) and the Moderna vaccine (100% and 96%, respectively); importantly, both vaccines were 96%–100% effective against severe, critical, or fatal disease, regardless of strain.(27, 28) In three studies from Canada, one demonstrated 79% effectiveness for mRNA vaccines against confirmed infection during a time when Alpha and Gamma represented most infections, while another two demonstrated 84% and 88% effectiveness, respectively, against symptomatic infection caused by Gamma/Beta.(29-31)
Individual studies specifically examining vaccine effectiveness against the Delta variant or conducted in the context of substantial circulation of Delta are summarized in Table 1a and as follows. Studies from the United Kingdom have noted effectiveness of the Pfizer-BioNTech vaccine against confirmed infection (79%) and symptomatic infection (88%), compared with the Alpha variant (92% and 93%, respectively).(23, 25) A study from Canada demonstrated 87% effectiveness against symptomatic illness caused by the Delta variant ≥7 days after receipt of the second dose of Pfizer-BioNTech vaccine, compared with 89% for the Alpha variant.(32)Data from Qatar demonstrated 54% effectiveness against symptomatic illness for the Pfizer-BioNTech vaccine compared with 85% for the Moderna vaccine.(33). Preliminary data from South Africa on the effectiveness of the Janssen vaccine showed 71% effectiveness against hospitalization when Delta variant was predominant, compared to 67% when Beta was predominant. Data from Israel also suggest decreased effectiveness of vaccines against infection and illness caused by Delta. The variability in vaccine effectiveness estimates between countries may in part reflect differences in study methodology, intervals used between vaccine doses, and timing of vaccine effectiveness assessments. Of note, the United Kingdom and Canada used prolonged intervals of 12–16 weeks between vaccine doses, which have been observed to induce higher immunogenicity and effectiveness (including in ages ≥80 years) (34-37). The most recent estimates from Israel and Qatar represent time points >6 months after initiating respective national vaccination campaigns and 2–5 months after prior assessments of vaccine effectiveness against the Alpha variant, with potential for waning immunity. Notably, in the United Kingdom, Canada, Qatar, South Africa, and Israel, vaccine effectiveness against hospitalization related to Delta was >90% and comparable to that observed with Alpha for all vaccines currently approved or authorized in the United States.(26, 32, 33)
Table 1a. Effectiveness of COVID-19 Vaccination Against SARS-CoV-2 Infection and Symptomatic Disease (Including Severe Disease and Hospitalization) Caused by the Delta Variant
| Country | Population | Vaccine | Outcome | Vaccine Effectiveness* |
| UK38 | General population ≥16 years | Pfizer-BioNTech | Symptomatic disease | 88%1(85-90) |
| Canada32 | General population ≥16 years | Pfizer-BioNTech | Symptomatic disease | 85%1(59-94) |
| UK (Scotland)25 | General population | Pfizer-BioNTech | SARS-CoV-2 infection | 79%1(75-82) |
| UK23 | General population | Pfizer-BioNTech | SARS-CoV-2 infection | 80%1(77-83) |
| United States39 | Healthcare workers, first responders, and other essential and frontline workers | Pfizer-BioNTech, Moderna, or Janssen | SARS-CoV-2 infection | 66%1(26-84) |
| United States40 | Health system members ≥12 years | Pfizer-BioNTech | SARS-CoV-2 infection | 75%2(71-78) |
| Hospitalization | 93%2(84-96) | |||
| Qatar33 | General population ≥12 years | Moderna | SARS-CoV-2 infection | 85%1(76-91) |
| Pfizer-BioNTech | SARS-CoV-2 infection | 54%1(44-61) | ||
| Moderna | Symptomatic disease | 86%1(71-94) | ||
| Pfizer-BioNTech | Symptomatic disease | 56%1(41-67) | ||
| Moderna | Severe, critical, or fatal disease | 100%1(41-100) | ||
| Pfizer-BioNTech | Severe, critical, or fatal disease | 90%1(61-98) | ||
| UK26 | Patients hospitalized following ED visit | Pfizer-BioNTech | Hospitalization | 96%1(86-99) |
*Only studies including estimates of vaccine effectiveness ≥7 days following a completed vaccination series of a COVID-19 vaccine currently approved or authorized for use in the United States are included here. For studies that examined variant-specific vaccine effectiveness against multiple variants of SARS-CoV-2, only estimates for effectiveness against the Delta variant are shown. The 95% confidence interval for each estimate of vaccine effectiveness is displayed in parentheses following the estimate.
1≥14 days after second dose
2≥7 days after second dose
In addition to preventing morbidity and mortality associated with COVID-19, currently approved or authorized vaccines also demonstrate effectiveness against asymptomatic SARS-CoV-2 infection. However, most studies of asymptomatic infection prevention were conducted in the context of circulation of different variants and the effectiveness of COVID-19 vaccines in preventing asymptomatic infection differs by variant and vaccine. In addition, infections identified in such studies as asymptomatic may simply have been identified prior to the infected person developing symptoms, i.e., these infections are presymptomatic rather than asymptomatic. Asymptomatic people are also less likely to be tested for SARS-CoV-2 infection in most settings and thus less likely to be captured in “real world” effectiveness studies.
Table 1b. Effectiveness of COVID-19 Vaccination Against Asymptomatic SARS-CoV-2 Infection When Different Variants Predominated
| Country | Population | Vaccine | Dominant Variant(s) | Vaccine Effectiveness* |
| Israel 24 | Healthcare workers | Pfizer-BioNTech | Alpha | 65%1(45-79) |
| United States (California) 41 | General population ≥18 years | Pfizer-BioNTech or Moderna | Epsilon, Alpha | 68%2(29-86) |
| United States42 | Preprocedural adult patients | Pfizer-BioNTech or Moderna | Ancestral strain | 80%3(56-91) |
| Qatar33 | General population ≥12 years | Moderna | Delta | 80%4(54-93) |
| Pfizer-BioNTech | Delta | 36%4(11-54) | ||
| Israel43 | Healthcare workers | Pfizer-BioNTech | Alpha | 86%5(69-93) |
| Israel21 | General population ≥16 years | Pfizer-BioNTech | Alpha | 92%5(91-92) |
| Israel19 | General population ≥16 years | Pfizer-BioNTech | Ancestral strain, Alpha | 90%5(83-94) |
*The 95% confidence interval for each estimate of vaccine effectiveness is displayed in parentheses following the estimate.
1≥11 days after second dose
2≥15 days after second dose
3≥0 days after second dose
4≥14 days after second dose
5≥7 days after second dose
Vaccine immunogenicity and effectiveness in immunocompromised people
Vaccination is particularly important for people with immunocompromising conditions, who are at increased risk of severe COVID-19 illness. However, current evidence suggests reduced protection from COVID-19 vaccines for many immunocompromised persons. Recent studies in several countries found significantly lower vaccine effectiveness among immunocompromised adults compared to those without immunocompromising conditions (44-46) (Table 2), although each study defined the immunocompromised population differently. Studies in the United States and Israel have also found that immunocompromised persons account for a high proportion (≥40%) of infections among fully vaccinated hospitalized persons. (46, 47)
Compared with those who are not immunocompromised, reduced antibody response to a two-dose primary series of mRNA COVID-19 vaccines has also been observed in specific groups of immunocompromised adults, including people receiving solid organ transplants (48-54); some people with cancer, particularly hematologic cancers (55, 56); some people receiving hemodialysis for kidney disease (57, 58); and people taking certain immunosuppressive medications (51, 53, 54, 59). While antibody measurement and threshold levels varied by study, a large proportion of immunocompromised persons overall had a measurable immune response after a two-dose series of mRNA vaccine, although some remained seronegative. The distribution of antibody response by immunocompromising condition in several recent studies is summarized in Figure 1.
Emerging data suggest an additional COVID-19 vaccine dose in immunocompromised people, typically administered at least 28 days after completion of the primary series, increases antibody response: in small observational studies of solid organ transplant recipients (60-63) or hemodialysis patients (64-66), 33%-54% of persons who had no detectable antibody response to an initial two-dose mRNA vaccine series developed an antibody response to an additional dose of a COVID-19 vaccine. A recently published randomized controlled trial demonstrated substantial increases in serologic immune response to a third dose of Moderna’s mRNA vaccine compared with placebo among solid organ transplant recipients who previously received a two-dose series of that vaccine.(67) While these studies evaluated serologic immune response to an additional vaccine dose, the clinical impact of an additional dose on acquisition, severity, and infectiousness of infections in fully vaccinated immunocompromised persons is not yet known.
Table 2. Effectiveness of COVID-19 Primary Series Vaccination Against SARS-CoV-2 Infection and Symptomatic Disease among Immunocompromised Persons
| Country | Population | Vaccine | Outcome | Dominant Variant(s) | Vaccine Effectiveness in IC Population | Vaccine Effectiveness in Comparison Population* |
| United States45 | Veterans ≥18 years taking immunosuppressive medications for inflammatory bowel disease | Pfizer-BioNTech or Moderna | SARS-CoV-2 infection | Unknown | 69%1(44-83) | No comparison |
| United States68 | Solid organ transplant recipients | Pfizer-BioNTech, Moderna, or Janssen | SARS-CoV-2 infection | Ancestral strain, Alpha | 81%2(50-95) | No comparison |
| Israel44 | General population ≥16 years | Pfizer-BioNTech | SARS-CoV-2 infection | Ancestral strain, Alpha | 71%1(37-87) | 90%(79-95) |
| Symptomatic disease | 75%1(44-88) | 94%(88-97) | ||||
| Qatar69 | Kidney transplant recipients | Pfizer-BioNTech or Moderna | SARS CoV-2 infection | Alpha, Beta | 47%2(0-74) | No comparison |
| Severe, critical, or fatal COVID-19 disease | 72%2(0-91) | |||||
| United States46 | Hospitalized patients ≥18 years | Pfizer-BioNTech or Moderna | Hospitalization | Ancestral strain, Alpha | 59%2(12-81) | 91%(86-95) |
IC: Immunocompromised
* In the Israeli study, the comparison is with overall vaccine effectiveness (i.e., vaccine effectiveness in the entire study population, including those with immunocompromising conditions). In the U.S. study, the comparison is with vaccine effectiveness among members of the study population without immunocompromising conditions.
The 95% confidence interval for each estimate of vaccine effectiveness is displayed in parentheses following the estimate.
1≥7 days after second dose
2≥14 days after second dose
Figure 1:
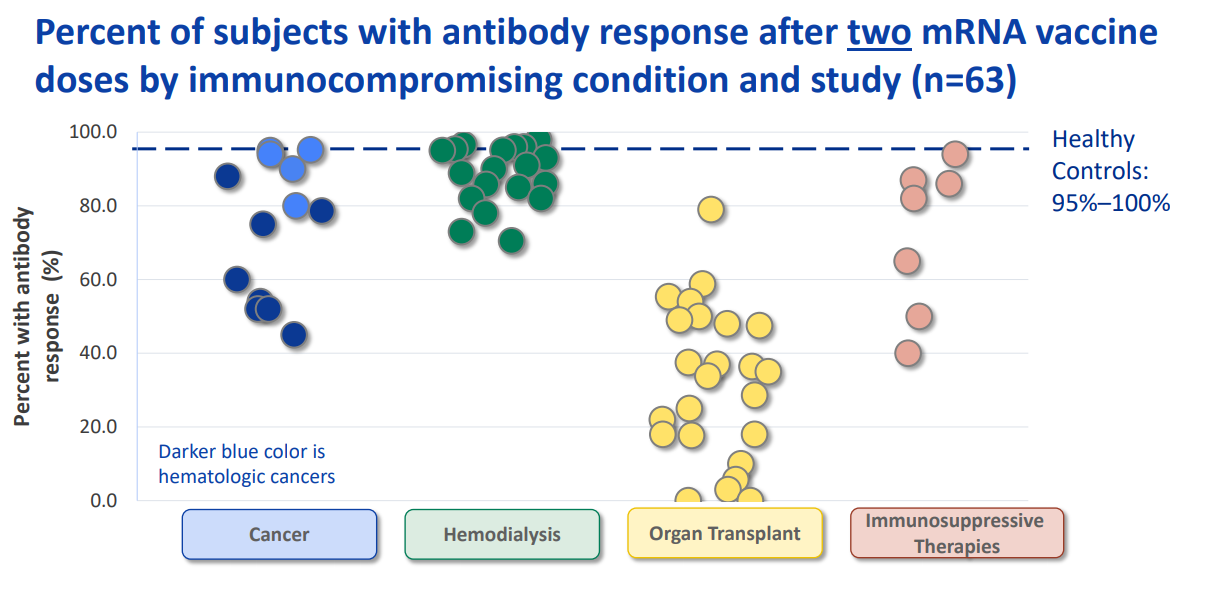
*The studies displayed in Figure 1 represent the results of a literature review conducted by the Advisory Committee on Immunization Practices’ COVID-19 Vaccines Work Group and are current as of July 21, 2021. Numerous additional studies of antibody response to COVID-19 vaccination in various immunocompromised populations have been published since that date and are not captured here.
Vaccine immunogenicity and effectiveness of heterologous (mixed) dosing regimens
Multiple small studies from Europe have examined the immunogenicity of a heterologous or ‘mixed’ series of COVID-19 vaccines. These studies found that receipt of a dose of AstraZeneca’s adenovirus vector vaccine followed by a dose of an mRNA vaccine (most frequently Pfizer-BioNTech) induced a robust immune response (70-72) and was at least as immunogenic as two doses of mRNA vaccines by most measures of immune response.(73-79) One study examined vaccine effectiveness of this heterologous series and estimated an effectiveness of 88% against any SARS-CoV-2 infection two weeks following the mRNA (second) dose.(80) Only one study examined a heterologous series in which the mRNA vaccine was the priming (first) dose; this study found that a dose of Pfizer-BioNTech vaccine followed by a dose of AstraZeneca vaccine did not achieve non-inferiority of immune response when compared with two doses of Pfizer-BioNTech.(81) A single study to date examined heterologous dosing with a primary mRNA vaccine series followed by a dose of the Janssen adenovirus vector COVID-19 vaccine in four subjects and noted substantially increased immune response against SARS-CoV-2 after the third dose.(82)
Vaccine-induced neutralizing antibody activity
Sera from mRNA COVID-19 vaccine (both Pfizer-BioNTech and Moderna) recipients have demonstrated minimal to large reductions in antibody neutralization activity against a variety of mutations, as reviewed in VIEW-Hub. Two related systematic reviews and meta-analyses have also been published (83, 84); however, these reviews do not include all available neutralization studies of the Delta variant with sera from people who received mRNA vaccines or the Janssen vaccine.(85-96) Across studies of VOCs, the greatest reductions were observed for Beta, followed by Gamma and Delta; reductions for Alpha were minimal. The E484K/Q and L452R mutations alone or in combination with other mutations in the receptor binding domain have been shown to account for the majority of the reduction in vaccine-induced neutralizing antibody activity for the Beta, Gamma, and Delta variants.(97-103) Alpha and Iota variants with E484K mutations, which have been detected in the United Kingdom, United States, and other countries, have shown further reductions in neutralization above Alpha and Iota alone, respectively.(87, 97, 104-109) For two-dose COVID-19 vaccines, multiple studies have shown greater neutralization against variants after the second dose (i.e. among fully vaccinated people) compared with after the first dose alone.(88, 91, 97, 98, 110-118)
Robust correlation has been demonstrated between vaccine efficacy and neutralizing antibody levels induced by different vaccines.(119, 120) Based on evidence from clinical trials, the correlate of protection, or antibody threshold providing protection against severe disease, has been estimated to be much lower than that required for protection against confirmed infection.(120) However, in the absence of an accepted antibody threshold that correlates with protection, it is difficult to fully predict how reduced neutralizing activity may affect COVID-19 vaccine effectiveness. Some variants may reduce neutralizing antibody levels to near or below the protective threshold, resulting in lowered vaccine efficacy, increased infections in vaccinated persons, and shortened duration of immunity, and others may not be significant.
Vaccine-induced cellular immunity
Several studies have assessed CD4+ and CD8+ T cell responses from Moderna or Pfizer-BioNTech vaccine recipients to the ancestral SARS-CoV-2 strain compared with the Alpha, Beta, Gamma, and Epsilon variants; these studies observed modest or no defects in cellular immune recognition of the variants.(112, 116, 121-126) Thus, cellular immunity may help limit disease severity in infections caused by variants that partially escape neutralizing antibodies. Variations in the genes encoding human leukocyte antigens have been observed to result in variation of the T cell response to specific SARS-CoV-2 variants, which may impact different subpopulations differently based on genetic prevalence of these variations.(127-132) There are currently no studies of vaccine-induced cellular immunity against the Delta variant.
Older adults and long-term care facility residents
Multiple studies have noted reduced vaccine effectiveness in older adults (≥60 years) (38, 133-135) or residents of long-term care facilities, compared with general population estimates.(136-138) Compared with younger individuals, persons aged >80 years have been noted to have reduced T-cell responses, lower neutralizing antibody levels, and less potential antibody diversity (somatic hypermutation), potentially giving this group increased risk for susceptibility to SARS-CoV-2 infection in vaccinated people. (139) Two studies have observed poor antibody response to the Pfizer-BioNTech vaccine among nursing home residents compared with staff (140, 141); one study noted 38% of nursing home residents had undetectable antibodies to the Beta variant at 2–4 weeks after the second dose of Pfizer-BioNTech vaccine, compared with 12% with Moderna vaccine.(140) Another study showed declining antibody levels among nursing home residents, with 72% of residents having undetectable neutralizing antibody levels at 6 months post-vaccination with Pfizer-BioNTech.(142)
Duration of protection
Immunogenicity of COVID-19 vaccines has been demonstrated out to 6–8 months after vaccination.(86, 143) At 2–3 months post vaccination, two studies have shown lower neutralizing titers, including against the Beta and Delta variants, for Janssen (an adenovirus vector vaccine) compared with the mRNA vaccines.(144, 145) Two studies have shown a combined impact of waning antibody levels and reduced neutralization of variants; six months after receiving the Moderna vaccine, neutralizing antibody levels were reduced but sufficient to protect against the ancestral strain, while about 50% of people had undetectable neutralization activity against Beta and Gamma compared with the ancestral strain.(146, 147) However, a small study of people 8 months after receiving the Janssen vaccine had minimal decline in neutralizing titers against Beta, Gamma, and Delta and there was evidence of expanded breadth of neutralizing antibody response against variants over this time period, likely through B cell maturation.(86) More evidence is still needed in this area, including understanding potential differences in the kinetics of immune response related to different vaccine platforms. One recent modeling study based on immunogenicity data predicted that vaccine effectiveness against symptomatic infection caused by the Delta variant may drop below 50% within the first year after vaccination for most current vaccines in use globally, while the majority are protected from severe illness.(148)
Six-month clinical efficacy for the Pfizer-BioNTech vaccine shows an overall efficacy against infection of 91% and 97% efficacy against severe illness.(149) However, a non-significant decrease of six percentage points was observed for every two months ≥7 days post-vaccination, from 96% at ≥7 days to <2 months, 90% at 2 to <4 months, and 84% at 4 to <6 months. Similar results for the Moderna vaccine have not yet been published, but data from the manufacturer cite 93% overall efficacy up to 6 months.
Several recent studies have noted decreases over time in the effectiveness of COVID-19 vaccines against SARS-CoV-2 infection. A study of U.S. long-term care residents, who were among the first groups in the United States to be vaccinated, found effectiveness of mRNA vaccination against infection declined from 75% in March–May 2021 to 53% in June–July 2021.(150) A study of adults in one U.S. state found a decline in vaccine effectiveness against SARS-CoV-2 infection from 92% the week of May 3, 2021 to 80% the week of July 19, 2021.(151) Two studies in large U.S. health systems examined mRNA vaccine effectiveness longitudinally from December 2020 and January 2021 through July 2021 and August 2021 and noted marked declines over this period (40, 152); similarly, a large population-based study in the UK identified decreases in effectiveness of Pfizer-BioNTech vaccination over 4-5 months following the second dose.(153) Observed changes in vaccine effectiveness against infection with SARS-CoV-2 may reflect reduced vaccine performance against the Delta variant, waning immunity from primary vaccination, or other unmeasured confounders. In addition, as people at the highest risk of SARS-CoV-2 infection were generally vaccinated first, observational studies of duration of immunity may be subject to confounding by risk status. Importantly, data as of July 2021 confirm sustained high effectiveness of full mRNA vaccination against COVID-19 hospitalization, even up to 6 months post-vaccination.(151, 154)
A retrospective cohort study in a large healthcare system in Israel noted a 2.3-fold increased risk for infection among fully vaccinated persons who were vaccinated with Pfizer-BioNTech in January vs. April 2021.(155) A similar study observed a higher rate (2.4% v. 1.1%, OR=2.2) of infection in fully vaccinated persons who received the second Pfizer-BioNTech dose ≥5 months ago compared with those who received it <5 months ago, with higher magnitude of difference with increasing age.(156)
Infections in fully vaccinated persons: clinical implications and transmission
As expected, because no vaccines is 100% effective, infections in fully vaccinated persons (e.g. breakthrough infections) have been observed, albeit at much lower rates than infections among unvaccinated persons; vaccine effectiveness against severe disease remains high. From January through June 2021, COVID-NET data from laboratory-confirmed COVID-19-associated hospitalizations in adults ≥18 years of age for whom vaccination status is known showed 3% of hospitalizations occurred in fully vaccinated persons. In general, symptoms and duration of illness in infections among fully vaccinated persons have been attenuated compared with cases among unvaccinated people.(157) CDC conducts nationwide monitoring of infections in fully vaccinated persons resulting in hospitalization or death. Among hospitalized or fatal cases reported to CDC as of August 30, 2021, 70% of hospitalized cases and 87% of fatal cases of COVID-19 in fully vaccinated persons were in persons aged 65 years or older. Infections in fully vaccinated persons may be associated with lower antibody levels compared with those who maintain protection, as shown in a study of fully vaccinated healthcare workers in Israel with infections caused by the Delta variant.(158) However, infection in a fully vaccinated person may boost immunity; four weeks after an outbreak in a long-term care facility, fully vaccinated residents who experienced SARS-CoV-2 infections were found to have significantly higher antibody levels than vaccinated individuals who did not experience SARS-CoV-2 infections.(159)
The proportions of VOCs observed among cases in fully vaccinated persons has been similar to that observed in CDC’s national genomic surveillance,(160) but interpretation of these data are challenging because of local variation and changes in variant proportions over time. An Israeli study of VOC infections in adults fully vaccinated with Pfizer-BioNTech vaccine compared with unvaccinated matched controls, during a time when Alpha was the dominant strain and Beta was detected in <1% of all specimens, found a higher proportion of Beta in fully vaccinated cases (matched odds ratio = 8.0) and a higher proportion of Alpha in partially vaccinated cases (matched odds ratio = 2.6), though small sample sizes, especially for Beta, were noted as a limitation.(161) Results of a study from Maryland showed that variants with E484K substitutions (e.g., Beta, Gamma) were associated with increased odds of SARS-CoV-2 infection (OR=2.0) in fully vaccinated persons and infection in fully vaccinated persons associated with hospitalization (OR=2.6), while L452R substitutions (e.g., Delta) were not.(162) However, a study from Houston, Texas observed that Delta caused a significantly higher rate of infections in fully vaccinated people compared with infections from other variants, but noted that only 6.5% of all COVID-19 cases occurred in fully vaccinated individuals(163); similar findings were noted in India.(96)
In studies conducted before the emergence of the Delta variant, data from multiple studies in different countries suggested that people vaccinated with mRNA COVID-19 vaccines who develop COVID-19 generally have a lower viral load than unvaccinated people.(157, 165-169) This observation may indicate reduced transmissibility, as viral load has been identified as a key driver of transmission.(170) Studies from multiple countries found significantly reduced likelihood of transmission to household contacts from people infected with SARS-CoV-2 who were previously vaccinated for COVID-19.(171-176) For the Delta variant, early data indicate vaccinated and unvaccinated persons infected with Delta have similar levels of viral RNA and culturable virus detected, indicating that some vaccinated people infected with the Delta variant of SARS-CoV-2 may be able to transmit the virus to others.(163, 164, 177-180) However, other studies have shown a more rapid decline in viral RNA and culturable virus in fully vaccinated people (96, 177, 180-182). One study observed that Delta infection in fully vaccinated persons was associated with significantly less transmission to contacts than persons who were unvaccinated or partially vaccinated.(181)
Together, these studies suggest that vaccinated people who become infected with Delta have potential to be less infectious than infected unvaccinated people. However, more data are needed to understand how viral shedding and transmission from fully vaccinated persons are affected by SARS-CoV-2 variants, time since vaccination, and other factors, particularly as transmission dynamics may vary based on the extent of exposure to the infected vaccinated person and the setting in which the exposure occurs. Additional data collection and studies are underway to understand the extent and duration of transmissibility of Delta variant SARS-CoV-2 in the United States and other countries.
Conclusions
COVID-19 vaccines currently approved or authorized in the United States have been shown to provide considerable protection against severe disease and death caused by COVID-19. These findings, along with the early evidence for reduced levels of viral mRNA and culturable virus in vaccinated people who acquire SARS-CoV-2 infection, suggest that any associated transmission risk is substantially reduced in vaccinated people: even for Delta, evidence suggests fully vaccinated people who become infected are infectious for shorter periods of time than unvaccinated people infected with Delta. While vaccine effectiveness against emerging and other SARS-CoV-2 variants will continue to be assessed, available evidence suggests that the COVID-19 vaccines approved or authorized in the United States offer substantial protection against hospitalization and death from emerging variants, including the Delta variant. Data suggest lower vaccine effectiveness against laboratory-confirmed illness and symptomatic disease caused by the Beta, Gamma, and Delta variants compared with the ancestral strain and Alpha variant. Early data also find some decline in vaccine effectiveness against SARS-CoV-2 infection over time, although in fall 2021, 9 months after the start of the U.S. COVID-19 vaccination program, vaccination remains highly protective against hospitalization with COVID-19.
Evidence suggests the U.S. COVID-19 vaccination program has substantially reduced the burden of disease in the United States by preventing serious illness in fully vaccinated people and interrupting chains of transmission. Vaccinated people can still become infected and have the potential to spread the virus to others, although at much lower rates than unvaccinated people. The risks of SARS-CoV-2 infection in fully vaccinated people are higher where community transmission of the virus is widespread. Current efforts to maximize the proportion of the U.S. population that is fully vaccinated against COVID-19 remain critical to ending the COVID-19 pandemic.
*Note: This brief summarizes evidence related to vaccines approved or authorized for emergency use in the United States. In specific circumstances, CDC guidance for fully vaccinated people can also be applied to COVID-19 vaccines that have been listed for emergency use by the World Health Organization (e.g. AstraZeneca/Oxford) and to some vaccines used for U.S. participants in COVID-19 vaccine trials.
References
Note: Preprints have not been peer-reviewed. They should not be regarded as conclusive, guide clinical practice/health-related behavior, or be reported in news media as established information.
- Lee G, Carr W, ACIP Evidence Based Recommendations Work Group. Updated Framework for Development of Evidence-Based Recommendations by the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018;67(45):1271-2.
- Centers for Disease Control and Prevention. SARS-CoV-2 Variant Classifications and Definitions [Available from: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html
- Corbett KS, Flynn B, Foulds KE, Francica JR, Boyoglu-Barnum S, Werner AP, et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N Engl J Med. 2020;383(16):1544-55.
- Mercado NB, Zahn R, Wegmann F, Loos C, Chandrashekar A, Yu J, et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586(7830):583-8.
- Vogel AB, Kanevsky I, Che Y, Swanson KA, Muik A, Vormehr M, et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403-16.
- Food and Drug Administration. Pfizer-BioNTech COVID-19 Vaccine. Vaccines and Related Biological Products Advisory Committee Briefing Document – Sponsor. https://www.fda.gov/media/144246/download.
- Food and Drug Administration. Moderna COVID-19 Vaccine. Vaccines and Related Biological Products Advisory Committee December 17, 2020 Meeting Briefing Document- Sponsor. https://www.fda.gov/media/144452/download.
- Food and Drug Administration. Moderna COVID-19 Vaccine. Vaccines and Related Biological Products Advisory Committee December 17, 2020 Meeting Briefing Document Addendum- Sponsor. https://www.fda.gov/media/144453/download.
- Food and Drug Administration. Janssen COVID-19 Vaccine. Vaccines and Related Biological Products Advisory Committee February 26, 2021 Meeting Briefing Document – Sponsor. https://www.fda.gov/media/146219/download.
- Food and Drug Administration. Janssen COVID-19 Vaccine. Vaccines and Related Biological Products Advisory Committee February 26, 2021 Meeting Briefing Document Addendum – Sponsor. https://www.fda.gov/media/146218/download.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603-15.
- Food and Drug Administration. Emergency Use Authorization (EUA) Amendment for an Unapproved Product Review Memorandum. https://www.fda.gov/media/148542/download.
- Sadoff J, Gray G, Vandebosch A, Cardenas V, Shukarev G, Grinsztejn B, et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;384(23):2187-201.
- Harder T, Koch J, Vygen-Bonnet S, Kulper-Schiek W, Pilic A, Reda S, et al. Efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 infection: interim results of a living systematic review, 1 January to 14 May 2021. Euro Surveill. 2021;26(28).
- Kow CS, Hasan SS. Real-world effectiveness of BNT162b2 mRNA vaccine: a meta-analysis of large observational studies. Inflammopharmacology. 2021;29(4):1075-90.
- Shapiro J, Dean NE, Madewell ZJ, Yang Y, Halloran ME, Longini I. Efficacy Estimates for Various COVID-19 Vaccines: What we Know from the Literature and Reports. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.05.20.21257461v2.
- Björk J, Inghammar M, Moghaddassi M, et al. Effectiveness of the BNT162b2 vaccine in preventing COVID-19 in the working age population – first results from a cohort study in Southern Sweden. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.04.20.21254636v1.
- Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021.
- Goldberg Y, Mandel M, Woodbridge Y, et al. Protection of previous SARS-CoV-2 infection is similar to that of BNT162b2 vaccine protection: A three-month nationwide experience from Israel. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.04.20.21255670v1.
- Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021.
- Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725-35.
- Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021.
- Regev-Yochay G, Amit S, Bergwerk M, et al. Decreased Infectivity Following BNT162b2 Vaccination: A prospective cohort study in Israel. The Lancet Regional Health – Europe. 2021;7(100150).
- Sheikh A, McMenamin J, Taylor B, Robertson C, Public Health S, the EIIC. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461-2.
- Stowe J, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against hospital admission with the Delta (B.1.617.2) variant. khubnet. 2021;https://khub.net/web/phe-national/public-library/-/document_library/v2WsRK3ZlEig/view/479607266.
- Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, Tang P, Hasan MR, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021.
- Abu-Raddad LJ, Chemaitelly H, Butt AA, National Study Group for C-V. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021.
- Chung H, He S, Nasreen S, et al. Effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe COVID-19 outcomes in Ontario, Canada. BMJ. 2021;Aug 20; 374:n1943.
- Nasreen S, Chung H, He S, et al. Effectiveness of COVID-19 vaccines against variants of concern in Ontario, Canada. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.06.28.21259420v2.
- Yassi A, Grant JM, Lockhart K, et al. Infection control, occupational and public health measures including mRNA-based vaccination against SARS-CoV-2 infections to protect healthcare workers from variants of concern: a 14-month observational study using surveillance data. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.05.21.21257600v1.
- Nasreen S CH, He S, et al. Effectiveness of COVID-19 vaccines against variants of concern in Ontario, Canada. medRxiv. 2021;https://doi.org/10.1101/2021.06.28.21259420.
- Tang P, Hasan MR, Chemaitelly H, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the Delta (B.1.617.2) variant in Qatar. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.08.11.21261885v1.
- Amirthalingam G, Lopez Bernal J, Andrews NJ, et al. Higher serological responses and increased vaccine effectiveness demonstrate the value of extended vaccine schedules in combatting COVID-19 in England. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.07.26.21261140v1.
- Carazo S, Talbot D, Boulianne N, et al. Single-dose mRNA vaccine effectiveness against SARS-CoV-2 in healthcare workers extending 16 weeks post-vaccination: a test-negative design from Quebec, Canada. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.07.19.21260445v1.
- Flaxman A, Marchevsky N, Jenkin D, et al. Tolerability and Immunogenicity After a Late Second Dose or a Third Dose of ChAdOx1 nCoV-19 (AZD1222). Preprints with The Lancet. 2021;https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3873839.
- Parry H, Bruton R, Stephens C, Amirthalingam G, Hallis B, Otter A, et al. Extended interval BNT162b2 vaccination enhances peak antibody generation in older people. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.05.15.21257017v1.
- Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088.
- Fowlkes A, Gaglani M, Groover K, et al. Effectiveness of COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Frontline Workers Before and During B.1.617.2 (Delta) Variant Predominance — Eight U.S. Locations, December 2020–August 2021. MMWR Morb Mortal Wkly Rep. 2021;ePub: 24 August 2021. DOI: http://dx.doi.org/10.15585/mmwr.mm7034e4http://dx.doi.org/10.15585/mmwr.mm7034e4.
- Tartof SY, Slezak JM, Fischer H, et al. Six-Month Effectiveness of BNT162B2 mRNA COVID-19 Vaccine in a Large US Integrated Health System: A Retrospective Cohort Study. Preprints with The Lancet. 2021;https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3909743.
- Andrejko K, Pry J, Myers JF, et al. Early evidence of COVID-19 vaccine effectiveness within the general population of California. MedRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.04.08.21255135v2.
- Tande AJ, Pollock BD, Shah ND, Farrugia G, Virk A, Swift M, et al. Impact of the COVID-19 Vaccine on Asymptomatic Infection Among Patients Undergoing Pre-Procedural COVID-19 Molecular Screening. Clin Infect Dis. 2021.
- Angel Y, Spitzer A, Henig O, et al. Association Between Vaccination With BNT162b2 and Incidence of Symptomatic and Asymptomatic SARS-CoV-2 Infections Among Health Care Workers. JAMA. 2021;325(24):2457-2465.
- Chodick G, Tene L, Rotem RS, Patalon T, Gazit S, Ben-Tov A, et al. The effectiveness of the two-dose BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. 2021.
- Khan N, Mahmud N. Effectiveness of SARS-CoV-2 Vaccination in a Veterans Affairs Cohort of Patients With Inflammatory Bowel Disease With Diverse Exposure to Immunosuppressive Medications. Gastroenterology. 2021;161(3):827-36.
- Tenforde MW, Patel MM, Ginde AA, et al. Effectiveness of SARS-CoV-2 mRNA Vaccines for Preventing Covid-19 Hospitalizations in the United States. medRxiv. 2021;https://doi.org/10.1101/2021.07.08.21259776
- Brosh-Nissimov T, Orenbuch-Harroch E, Chowers M, Elbaz M, Nesher L, Stein M, et al. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect. 2021.
- Boyarsky BJ, Chiang TP, Ou MT, Werbel WA, Massie AB, Segev DL, et al. Antibody Response to the Janssen COVID-19 Vaccine in Solid Organ Transplant Recipients. Transplantation. 2021;105(8):e82-e3.
- Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA. 2021.
- Chavarot N, Ouedrani A, Olivier M, et al. Poor Anti-SARS-CoV-2 Humoral and T-cell Responses After 2 Injections of mRNA Vaccine in Kidney Transplant Recipients Treated with Belatacept. Transplantation. 2021;105(9):e94-e5.
- Grupper A, Rabinowich L, Schwartz D, Schwartz IF, Ben-Yehoyada M, Shashar M, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021.
- Itzhaki Ben Zadok O, Shaul AA, Ben-Avraham B, Yaari V, Ben Zvi H, Shostak Y, et al. Immunogenicity of the BNT162b2 mRNA vaccine in heart transplant recipients – a prospective cohort study. Eur J Heart Fail. 2021.
- Rabinowich L, Grupper A, Baruch R, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75:435-8.
- Rozen-Zvi B, Yahav D, Agur T, Zingerman B, Ben-Zvi H, Atamna A, et al. Antibody response to mRNA SARS-CoV-2 vaccine among kidney transplant recipients – Prospective cohort study. Clin Microbiol Infect. 2021.
- Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID-19 Vaccine in Patients with Chronic Lymphocytic Leukemia. Blood. 2021.
- Monin L, Laing AG, Munoz-Ruiz M, McKenzie DR, Del Molino Del Barrio I, Alaguthurai T, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021.
- Broseta JJ, Rodriguez-Espinosa D, Rodriguez N, Mosquera MDM, Marcos MA, Egri N, et al. Humoral and Cellular Responses to mRNA-1273 and BNT162b2 SARS-CoV-2 Vaccines Administered to Hemodialysis Patients. Am J Kidney Dis. 2021.
- Simon B, Rubey H, Treipl A, et al. Hemodialysis Patients Show a Highly Diminished Antibody Response after COVID-19 mRNA Vaccination Compared to Healthy Controls. Nephrol Dial Transplant. 2021:1-8.
- Boyarsky BJ, Ruddy JA, Connolly CM, Ou MT, Werbel WA, Garonzik-Wang JM, et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021.
- Charmetant X, Espi M, Benotmane I, et al. Comparison of infected and vaccinated transplant recipients highlights the role of Tfh and neutralizing IgG in COVID-19 protection. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.07.22.21260852v1.
- Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three Doses of an mRNA Covid-19 Vaccine in Solid-Organ Transplant Recipients. N Engl J Med. 2021;385(7):661-2.
- Schrezenmeier E, Rincon-Arevalo H, Stefanski AL, et al. B and T cell responses after a third dose of SARS-CoV-2 vaccine in Kidney Transplant Recipients. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.08.12.21261966v2.full.
- Werbel WA, Boyarsky BJ, Ou MT, Massie AB, Tobian AAR, Garonzik-Wang JM, et al. Safety and Immunogenicity of a Third Dose of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Ann Intern Med. 2021.
- Ducloux D, Colladant M, Chabannes M, Yannaraki M, Courivaud C. Humoral response after 3 doses of the BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney Int. 2021;100(3):702-4.
- Espi M, Charmetant X, Barba T, et al. Justification, safety, and efficacy of a third dose of mRNA vaccine in maintenance hemodialysis patients: a prospective observational study. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.07.02.21259913v1.
- Longlune N, Nogier MB, Miedouge M, Gabilan C, Cartou C, Seigneuric B, et al. High immunogenicity of a messenger RNA based vaccine against SARS-CoV-2 in chronic dialysis patients. Nephrol Dial Transplant. 2021.
- Hall VG, Ferreira VH, Ku T, Ierullo M, Majchrzak-Kita B, Chaparro C, et al. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients. N Engl J Med. 2021.
- Aslam S, Adler E, Mekeel K, Little SJ. Clinical effectiveness of COVID-19 vaccination in solid organ transplant recipients. Transpl Infect Dis. 2021:e13705.
- Chemaitelly H, AlMukdad S, Joy JP, et al. SARS-CoV-2 vaccine effectiveness in immunosuppressed kidney transplant recipients. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.08.07.21261578v1.full.
- Behrens GM, Cossmann A, Stankov MV, Nehlmeier I, Kempf A, Hoffmann M, et al. SARS-CoV-2 delta variant neutralisation after heterologous ChAdOx1-S/BNT162b2 vaccination. Lancet. 2021.
- Borobia AM, Carcas AJ, Perez-Olmeda M, Castano L, Bertran MJ, Garcia-Perez J, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398(10295):121-30.
- Normark J, Vikstrom L, Gwon YD, Persson IL, Edin A, Bjorsell T, et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 Vaccination. N Engl J Med. 2021.
- Rose R, Neumann, F., Grobe, O., et al. Heterologous immunisation with vector vaccine as prime followed by mRNA vaccine as boost leads to humoral immune response against SARS-CoV-2, which is comparable to that according to a homologous mRNA vaccination scheme. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.07.09.21260251v1.
- Groß R, Zanoni M, Seidel A, et al. Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.05.30.21257971v2.
- Schmidt T, Klemis V, Schub D, Mihm J, Hielscher F, Marx S, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med. 2021.
- Hillus D, Schwarz T, Tober-Lau P, Vanshylla K, Hastor H, Thibeault C, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir Med. 2021.
- Tenbusch M, Schumacher S, Vogel E, Priller A, Held J, Steininger P, et al. Heterologous prime-boost vaccination with ChAdOx1 nCoV-19 and BNT162b2. Lancet Infect Dis. 2021.
- Barros-Martins J, Hammerschmidt SI, Cossmann A, Odak I, Stankov MV, Morillas Ramos G, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021.
- Brehm TT, Thompson M, Ullrich F, et al. Low SARS-CoV-2 infection rate and high vaccine-induced immunity among German healthcare workers at the end of the third wave of the COVID-19 pandemic. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.08.02.21260667v1.
- Gram MA, Nielsen J, Schelde AB, et al. Vaccine effectiveness when combining the ChAdOx1 vaccine as the first dose with an mRNA COVID-19 vaccine as the second dose. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.07.26.21261130v1.
- Liu X, Shaw RH, Stuart ASV, Greenland M, Aley PK, Andrews NJ, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021.
- Iketani S, Liu L, Nair MS, et al. A third COVID-19 vaccine shot markedly boosts neutralizing antibody potency and breadth. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.08.11.21261670v1.
- Chen X, Chen Z, Azman AS, et al. Comprehensive mapping of neutralizing antibodies against SARS-CoV-2 variants induced by natural infection or vaccination. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.05.03.21256506v1.
- Noori M, Nejadghaderi SA, Arshi S, Carson-Chahhoud K, Ansarin K, Kolahi AA, et al. Potency of BNT162b2 and mRNA-1273 vaccine-induced neutralizing antibodies against severe acute respiratory syndrome-CoV-2 variants of concern: A systematic review of in vitro studies. Rev Med Virol. 2021:e2277.
- Arora P, Kempf A, Nehlmier I, et al. Increased lung cell entry of B.1.617.2 and evasion of antibodies induced by infection and BNT162b2 vaccination. bioRxiv. 2021;https://www.biorxiv.org/content/10.1101/2021.06.23.449568v1.
- Barouch DH, Stephenson KE, Sadoff J, et al. Durable Humoral and Cellular Immune Responses Following Ad26.COV2.S Vaccination for COVID-19. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.07.05.21259918v1.
- Choi A, Koch M, Wu K, et al. Serum Neutralizing Activity of mRNA-1273 against SARS-CoV-2 Variants. bioRxiv. 2021;https://www.biorxiv.org/content/10.1101/2021.06.28.449914v1.
- Davis C, Logan N, Tyson G, et al. Reduced neutralisation of the Delta (B.1.617.2) SARS-CoV-2 variant of concern following vaccination. MedRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.06.23.21259327v1.
- Edara VV, Pinsky BA, Suthar MS, Lai L, Davis-Gardner ME, Floyd K, et al. Infection and Vaccine-Induced Neutralizing-Antibody Responses to the SARS-CoV-2 B.1.617 Variants. N Engl J Med. 2021.
- Jongeneelen M, Kaszas K, Veldman D, et al. Ad26.COV2.S elicited neutralizing activity against Delta and other SARS-CoV-2 variants of concern. bioRxiv. 2021;https://www.biorxiv.org/content/10.1101/2021.07.01.450707v1.
- Liu C, Ginn HM, Dejnirattisai W, Supasa P, Wang B, Tuekprakhon A, et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021.
- Liu J, Liu Y, Xia H, Zou J, Weaver SC, Swanson KA, et al. BNT162b2-elicited neutralization of B.1.617 and other SARS-CoV-2 variants. Nature. 2021.
- Lustig Y, Zuckerman N, Nemet I, Atari N, Kliker L, Regev-Yochay G, et al. Neutralising capacity against Delta (B.1.617.2) and other variants of concern following Comirnaty (BNT162b2, BioNTech/Pfizer) vaccination in health care workers, Israel. Euro Surveill. 2021;26(26).
- Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596(7871):276-80.
- Wall EC, Wu M, Harvey R, Kelly G, Warchal S, Sawyer C, et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397(10292):2331-3.
- Mlcochova P KS, Dhar MS, et al. . SARS-CoV-2 B.1.617.2 Delta variant emergence and vaccine breakthrough. Research Square. 2021 https://www.researchsquare.com/article/rs-637724/v1.
- Collier DA, De Marco A, Ferreira I, Meng B, Datir R, Walls AC, et al. Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature. 2021.
- Garcia-Beltran WF, Lam EC, St Denis K, Nitido AD, Garcia ZH, Hauser BM, et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021.
- Jangra S, Ye C, Rathnasinghe R, Stadlbauer D, Personalized Virology Initiative study g, Krammer F, et al. SARS-CoV-2 spike E484K mutation reduces antibody neutralisation. Lancet Microbe. 2021.
- Lucas C, Vogels CBF, Yildirim I, et al. Impact of circulating SARS-CoV-2 variants on mRNA vaccine-induced immunity in uninfected and previously infected individuals. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.07.14.21260307v1.
- Tada T, Dcosta BM, Samanovic MI, Herati RS, Cornelius A, Zhou H, et al. Convalescent-Phase Sera and Vaccine-Elicited Antibodies Largely Maintain Neutralizing Titer against Global SARS-CoV-2 Variant Spikes. mBio. 2021;12(3):e0069621.
- Tada T, Zhou H, Dcosta BM, et al. SARS-CoV-2 Lambda Variant Remains Susceptible to Neutralization by mRNA Vaccine-elicited Antibodies and Convalescent Serum. BioRxiv. 2021;https://www.biorxiv.org/content/10.1101/2021.07.02.450959v1.
- Wang P, Nair MS, Liu L, Iketani S, Luo Y, Guo Y, et al. Antibody Resistance of SARS-CoV-2 Variants B.1.351 and B.1.1.7. Nature. 2021.
- Annavajhala MK, Mohri H, Zucker JE, Sheng Z, Wang P, Gomez-Simmonds A, et al. A Novel SARS-CoV-2 Variant of Concern, B.1.526, Identified in New York. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.02.23.21252259v4.
- Carreno JM, Alshammary H, Singh G, et al. Reduced neutralizing activity of post-SARS-CoV-2 vaccination serum against variants B.1.617.2, B.1.351, B.1.1.7+E484K and a sub-variant of C.37. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.07.21.21260961v1.
- Liu Y, Liu J, Xia H, Zhang X, Zou J, Fontes-Garfias CR, et al. BNT162b2-Elicited Neutralization against New SARS-CoV-2 Spike Variants. N Engl J Med. 2021.
- West AP WJ, Wang JC, et al. Detection and characterization of the SARS-CoV-2 lineage B.1.526 in New York. bioRxiv. 2021;https://www.biorxiv.org/content/10.1101/2021.02.14.431043v3.
- Wu K, Werner AP, Koch M, Choi A, Narayanan E, Stewart-Jones GBE, et al. Serum Neutralizing Activity Elicited by mRNA-1273 Vaccine. N Engl J Med. 2021.
- Zhou H, Dcosta B, Samanovic M, et al. B.1.526 SARS-CoV-2 variants identified in New York City are neutralized by vaccine-elicited and therapeutic monoclonal antibodies. bioRxiv. 2021;https://www.biorxiv.org/content/10.1101/2021.03.24.436620v1.full.pdf.
- Alenquer M, Ferreira F, Lousa D, et al. Amino acids 484 and 494 of SARS-CoV-2 spike are hotspots of immune evasion affecting antibody but not ACE2 binding. bioRxiv. 2021;https://www.biorxiv.org/content/10.1101/2021.04.22.441007v2.
- Becker M, Dulovic A, Junker D, Ruetalo N, Kaiser PD, Pinilla YT, et al. Immune response to SARS-CoV-2 variants of concern in vaccinated individuals. Nat Commun. 2021;12(1):3109.
- Geers D, Shamier MC, Bogers S, den Hartog G, Gommers L, Nieuwkoop NN, et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol. 2021;6(59).
- Marot S, Malet I, Leducq V, Abdi B, Teyssou E, Soulie C, et al. Neutralization heterogeneity of United Kingdom and South-African SARS-CoV-2 variants in BNT162b2-vaccinated or convalescent COVID-19 healthcare workers. Clin Infect Dis. 2021.
- Planas D, Bruel T, Grzelak L, Guivel-Benhassine F, Staropoli I, Porrot F, et al. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat Med. 2021;27(5):917-24.
- Shen X, Tang H, McDanal C, Wagh K, Fischer W, Theiler J, et al. SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe. 2021.
- Skelly D, Harding A, Gilbert-Jaramillo J, et al. Two doses of SARS-CoV-2 vaccination induce robust immune responses to emerging SARS-CoV-2 variants of concern. Nature Communications. 2021;12(5061):1-12.
- Stamatatos L, Czartoski J, Wan YH, Homad LJ, Rubin V, Glantz H, et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021.
- Supasa P, Zhou D, Dejnirattisai W, Liu C, Mentzer AJ, Ginn HM, et al. Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell. 2021.
- Earle KA, Ambrosino DM, Fiore-Gartland A, Goldblatt D, Gilbert PB, Siber GR, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39(32):4423-8.
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205-11.
- Gallagher KME, Leick MB, Larson RC, Berger TR, Katsis K, Yam JY, et al. SARS -CoV-2 T-cell immunity to variants of concern following vaccination. bioRxiv. 2021.
- Lilleri D, Vassaniti I, Bergami F, et al. SARS-CoV-2 mRNA vaccine BNT162b2 elicited a robust humoral and cellular response against SARS-CoV-2 variants. Research Square. 2021;https://www.researchsquare.com/article/rs-396284/v1.
- Neidleman J, Luo X, McGregor M, et al. mRNA vaccine-induced SARS-CoV-2-specific T cells recognize B.1.1.7 and B.1.351 variants but differ in longevity and homing properties depending on prior infection status. bioRxiv. 2021;https://www.biorxiv.org/content/10.1101/2021.05.12.443888v2.
- Stankov MV, Cossmann A, Bonifacius A, Dopfer-Jablonka A, Ramos GM, Godecke N, et al. Humoral and cellular immune responses against SARS-CoV-2 variants and human coronaviruses after single BNT162b2 vaccination. Clin Infect Dis. 2021:1-9.
- Tarke A, Sidney J, Methot N, et al. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Reports Medicine. 2021;2(7):1-12.
- Woldemeskel BA, Garliss CC, Blankson JN. SARS-CoV-2 mRNA vaccines induce broad CD4+ T cell responses that recognize SARS-CoV-2 variants and HCoV-NL63. J Clin Invest. 2021;131(10).
- Motozono C, Toyoda M, Zahradnik J, et al. An emerging SARS-CoV-2 mutant evading cellular immunity and increasing viral infectivity. bioRxiv. 2021;https://www.biorxiv.org/content/10.1101/2021.04.02.438288v1.
- Pretti MAM, Galvani RG, Farias AS, et al. New SARS-CoV-2 lineages could evade CD8+ T-cells response. bioRxiv. 2021;https://www.biorxiv.org/content/10.1101/2021.03.09.434584v2.
- Reynolds CJ, Pade C, Gibbons JM, Butler DK, Otter AD, Menacho K, et al. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science. 2021.
- Dolton G, Rius C, Hasan MS, et al. Emergence of immune escape at dominant SARS-CoV-2 killer T-cell epitope. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.06.21.21259010v2.
- Agerer B, Koblischke M, Gudipati V, Montano-Gutierrez LF, Smyth M, Popa A, et al. SARS-CoV-2 mutations in MHC-I-restricted epitopes evade CD8(+) T cell responses. Sci Immunol. 2021;6(57).
- Buckley PR, Lee CH, Pinho MP, et al. HLA-dependent variation in SARS-CoV-2 CD8+ T cell cross-reactivity with human coronaviruses. bioRxiv. 2021;https://www.biorxiv.org/content/10.1101/2021.07.17.452778v1.
- Aran D. Estimating real-world COVID-19 vaccine effectiveness in Israel using aggregated counts. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.02.05.21251139v3.
- Gomes D, Beyerlein A, Katz K, et al. Is the BioNTech-Pfizer COVID-19 vaccination effective in elderly populations? Results from population data from Bavaria, Germany. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.08.19.21262266v1.
- Mason T, Whitston M, Hodgson J, et al. Effects of BNT162b2 mRNA vaccine on Covid-19 infection and hospitalisation among older people: matched case control study for England. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.04.19.21255461v1.
- Cavanaugh AM, Fortier S, Lewis P, Arora V, Johnson M, George K, et al. COVID-19 Outbreak Associated with a SARS-CoV-2 R.1 Lineage Variant in a Skilled Nursing Facility After Vaccination Program – Kentucky, March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(17):639-43.
- Emborg H, Valentiner-Branth P, Schelde AB, et al. Vaccine effectiveness of the BNT162b2 mRNA COVID-19 vaccine against RT-PCR confirmed SARS-CoV-2 infections, hospitalisations and mortality in prioritised risk groups. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.05.27.21257583v1.
- Moustsen-Helms I, Emborg HD, Nielsen J, et al. Vaccine effectiveness after 1st and 2nd dose of the BNT162b2 mRNA Covid-19 Vaccine in long-term care facility residents and healthcare workers – a Danish cohort study medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.03.08.21252200v1(March 24, 2021).
- Collier DA, Ferreira I, Kotagiri P, Datir RP, Lim EY, Touizer E, et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596(7872):417-22.
- Abe KT, Hu Q, Mozafarihashjin M, et al. Neutralizing antibody responses to SARS-CoV-2 variants in vaccinated Ontario long-term care home residents and workers. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.08.06.21261721v1.full.pdf.
- Pannus P, Neven, K.Y., De Craeye, S., et al. Poor antibody response to BioNTech/Pfizer COVID-19 vaccination in SARS-CoV-2 naïve residents of nursing homes. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.06.08.21258366v1.
- Canaday DH, Oyebanji OA, Keresztesy D, et al. Significant reduction in humoral immunity among healthcare workers and nursing home residents 6 months after COVID-19 BNT162b2 mRNA vaccination. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.08.15.21262067v1.full.pdf.
- Doria-Rose N, Suthar MS, Makowski M, O’Connell S, McDermott AB, Flach B, et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19. N Engl J Med. 2021;384(23):2259-61.
- Tada T, Zhou H, Samanovic M, et al. Comparison of Neutralizing Antibody Titers Elicited by mRNA and Adenoviral Vector Vaccine against SARS-CoV-2 Variants. bioRxiv. 2021;https://doi.org/10.1101/2021.07.19.452771
- Naranbhai V, Garcia-Beltran, W.F., Berrios Mairena, C., et al. . Immunogenicity of mRNA-1273, BNT162b2 and Ad26.COV2.S COVID-19 vaccines. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.07.18.21260732v1.
- Pegu A, O’Connell S, Schmidt SD, O’Dell S, Talana CA, Lai L, et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science. 2021.
- Wu K, Choi A, Koch M, et al. Preliminary Analysis of Safety and Immunogenicity of a SARS-CoV-2 Variant Vaccine Booster. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.05.05.21256716v1.
- Cromer D, Steain M, Reynaldi A, et al. SARS-CoV-2 variants: levels of neutralisation required for protective immunity. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.08.11.21261876v1.
- Thomas SJ, Moreira ED, Kitchin N, et al. Six Month Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.07.28.21261159v1.
- Nanduri S, Pilishvili T, Derado G, et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines in Preventing SARS-CoV-2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant — National Healthcare Safety Network, March 1–August 1, 2021. MMWR Morb Mortal Wkly Rep. 2021;ePub: 18 August 2021. DOI: http://dx.doi.org/10.15585/mmwr.mm7034e3.
- Rosenberg ES, Holtgrave DR, Dorabawila V, et al. New COVID-19 Cases and Hospitalizations Among Adults, by Vaccination Status — New York, May 3–July 25, 2021. MMWR Morb Mortal Wkly Rep. 2021;ePub: 18 August 2021. DOI: http://dx.doi.org/10.15585/mmwr.mm7034e1.
- Puranik A, Lenehan PJ, Silvert E, et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.08.06.21261707v3.
- Pouwels KB, Pritchard E, Matthews PC, et al. Impact of Delta on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. 2021;https://www.ndm.ox.ac.uk/files/coronavirus/covid-19-infection-survey/finalfinalcombinedve20210816.pdf.
- Tendforde MW, Self WH, Naioti EA, et al. Sustained Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Associated Hospitalizations Among Adults — United States, March–July 2021. MMWR Morb Mortal Wkly Rep. 2021;ePub: 18 August 2021. DOI: http://dx.doi.org/10.15585/mmwr.mm7034e2.
- Mizrahi B, Lotan R, Kalkstein N, et al. Correlation of SARS-CoV-2 Breakthrough Infections to Time-from-vaccine; Preliminary Study. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.07.29.21261317v1.full.
- Israel A, Merzon E, Schäffer AA, et al. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection in a large cohort. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.08.03.21261496v1.
- Thompson MG, Burgess JL, Naleway AL, Tyner H, Yoon SK, Meece J, et al. Prevention and Attenuation of Covid-19 with the BNT162b2 and mRNA-1273 Vaccines. N Engl J Med. 2021;385(4):320-9.
- Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N Engl J Med. 2021.
- Muller L, Andree M, Ostermann PN, et al. SARS-CoV-2 infection in fully vaccinated individuals of old age strongly boosters the humoral immune response. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.07.19.21260563v1.
- Centers for Disease Control and Prevention. COVID-19 Vaccine Breakthrough Infections Reported to CDC — United States, January 1–April 30, 2021 [Available from: https://www.cdc.gov/mmwr/volumes/70/wr/mm7021e3.htm?s_cid=mm7021e3_w.
- Kustin T, Harel N, Finkel U, Perchik S, Harari S, Tahor M, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021.
- Feder KA, Patel A, Vepachedu VR, et al. Association of E484K and L452R spike protein mutations with SARS-CoV-2 infection in vaccinated persons—Maryland, January – May 2021. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.07.29.21261006v2.
- Musser JM, Christensen PA, Olsen RJ, et al. Delta variants of SARS-CoV-2 cause significantly increased vaccine breakthrough COVID-19 cases in Houston, Texas. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.07.19.21260808v2.
- Brown CM, Vostok J, Johnson H, Burns M, Gharpure R, Sami S, et al. Outbreak of SARS-CoV-2 Infections, Including COVID-19 Vaccine Breakthrough Infections, Associated with Large Public Gatherings – Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(31):1059-62.
- Jones NK, Rivett L, Seaman S, Samworth RJ, Warne B, Workman C, et al. Single-dose BNT162b2 vaccine protects against asymptomatic SARS-CoV-2 infection. Elife. 2021;10.
- Levine-Tiefenbrun M, Yelin I, Katz R, Herzel E, Golan Z, Schreiber L, et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat Med. 2021;27(5):790-2.
- McEllistrem MC, Clancy CJ, Buehrle DJ, Lucas A, Decker BK. Single dose of a mRNA SARS-CoV-2 vaccine is associated with lower nasopharyngeal viral load among nursing home residents with asymptomatic COVID-19. Clin Infect Dis. 2021.
- Petter E, Mor O, Zuckerman N, et al. Initial real world evidence for lower viral load of individuals who have been vaccinated by BNT162b2. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.02.08.21251329v1.
- Abu-Raddad LJ, Chemaitelly H., Ayoub H.H., et al. Effect of vaccination and of prior infection on infectiousness of vaccine breakthrough infections and reinfections. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.07.28.21261086v1.
- Marks M, Millat-Martinez P, Ouchi D, Roberts CH, Alemany A, Corbacho-Monne M, et al. Transmission of COVID-19 in 282 clusters in Catalonia, Spain: a cohort study. Lancet Infect Dis. 2021.
- de Gier B, Andeweg S, Joosten R, Ter Schegget R, Smorenburg N, van de Kassteele J, et al. Vaccine effectiveness against SARS-CoV-2 transmission and infections among household and other close contacts of confirmed cases, the Netherlands, February to May 2021. Euro Surveill. 2021;26(31).
- Harris RJ, Hall JA, Zaidi A, Andrews NJ, Dunbar JK, Dabrera G. Effect of Vaccination on Household Transmission of SARS-CoV-2 in England. N Engl J Med. 2021;385(8):759-60.
- Layan M, Gilboa M, Gonen T. Impact of BNT162b2 vaccination and isolation on SARS-CoV-2 transmission in Israeli households: an observational study. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.07.12.21260377v1.
- Prunas O, Warren JL, Crawford FW, et al. Vaccination with BNT162b2 reduces transmission of SARS-CoV-2 to household contacts in Israel. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.07.13.21260393v1.
- Salo J, Hagg M, Kortelainen M, et al. The indirect effect of mRNA-based Covid-19 vaccination on unvaccinated household members. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.05.27.21257896v2.
- Shah A, Gribben C, Bishop J, et al. Effect of vaccination on transmission of COVID-19: an observational study in healthcare workers and their households. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.03.11.21253275v1.
- Chia PY, Ong SWX, Chiew C, et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine-breakthrough infections: a multi-center cohort study. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.07.28.21261295v1.
- Griffin JB, Haddix M, Danza P, et al. SARS-CoV-2 Infections and Hospitalizations Among Persons Aged ≥16 Years, by Vaccination Status — Los Angeles County, California, May 1–July 25, 2021. MMWR Morb Mortal Wkly Rep. 2021;ePub: 24 August 2021. DOI: http://dx.doi.org/10.15585/mmwr.mm7034e5.
- Riemersma KK, Grogan BE, Kita-Yarbro A, et al. Shedding of Infectious SARS-CoV-2 Despite Vaccination when the Delta Variant is Prevalent – Wisconsin, July 2021. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.07.31.21261387v4.
- Shamier MC, Tostmann A, Bogers S. Virological characteristics of SARS-CoV-2 vaccine breakthrough infections in health care workers. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.08.20.21262158v1.
- Kang M, Xin H, Yuan J. Transmission dynamics and epidemiological characteristics of Delta variant infections in China. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.08.12.21261991v1.
- Ong SWX, Chiew CJ, Ang LW, et al. Clinical and Virological Features of SARS-CoV-2 Variants of Concern: A Retrospective Cohort Study Comparing B.1.1.7 (Alpha), B.1.315 (Beta), and B.1.617.2 (Delta). Preprints with The Lancet. 2021;https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3861566.