Summary of the 2011-2012 Influenza Season
- What was the 2011-2012 flu season like?
- When did the 2011-2012 flu season peak?
- How severe was the season?
- How is severity characterized?
- What flu viruses circulated this season?
- How effective was the 2011-2012 seasonal flu vaccine?
- Can VE be higher for flu vaccine? Why is flu vaccine recommended when VE for the 2011-2012 season vaccine was about 50%?
- What factors influence flu vaccine effectiveness?
- What did CDC do to monitor effectiveness of flu vaccines for the 2011-2012 season?
- What did CDC do to monitor antiviral resistance in the United States during the 2011-12 season?
- Were infections with unusual influenza viruses detected in 2011-2012?
- Publications
Season Summary Reports
What was the 2011-2012 flu season like?
In comparison to other seasons, the 2011-2012 season set a new record for the lowest and shortest peak of influenza-like illness. The season began late and was mild compared to most previous seasons for which surveillance data is available.
Flu seasons are unpredictable in a number of ways, including when they begin, how severe they are, how long they last, which viruses will spread, and whether the viruses in the vaccine match flu viruses that are circulating.
Additional information about flu activity during the 2011-2012 season can be found in 2011-2012 Flu Season Draws to a Close and in the MMWR article Update: Influenza Activity — United States, 2011-12 Season, and Composition of the 2012-13 Influenza Vaccine.
When did the 2011-2012 flu season peak?
Influenza-like illness (ILI) in the United States typically begins to increase in late December or early January and peaks in February most commonly. During the 2011-2012 season, ILI remained low through February and did not peak until mid-March. Also, ILI reached the baseline for only 1 week during the season. This is the first time since CDC started this kind of ILI surveillance that the percentage of patient visits for ILI was elevated for only one week of the season. In past seasons, ILI has remained above baseline for between 8 and 20 weeks, with an average of 13 weeks at or above baseline each season since this type of surveillance began in 1997-1998. In terms of ILI, this not only the shortest time at or above baseline, but it’s also the lowest ‘peak’ ever recorded. (A more detailed description of influenza-like illness surveillance in the United States is available at Overview of Influenza Surveillance in the United States.)
How severe was the season?
Severity of a season is determined by a number of things including the following: the percentage of deaths resulting from pneumonia or influenza, the number of influenza-associated pediatric deaths reported, rates of influenza-associated hospitalizations and the percentage of visits to outpatient clinics for ILI.
Percentage of deaths resulting from pneumonia or influenza
During the 2011–12 influenza season, the percentage of deaths attributed to pneumonia and influenza was elevated above baseline for only 1 week. (For comparison, from the 2008–09 season through the 2010–11 season, the total number of consecutive weeks when the percentage of deaths attributed to pneumonia and influenza was at or above the baseline ranged from 3 to 13.)
Number of influenza-associated pediatric deaths
As of September 2014, 37 pediatric deaths occurring during the 2011-2012 season had been reported to CDC. Since 2004 the number of pediatric deaths has ranged from a low of 37 deaths during the 2011-2012 season, to a high of 288 deaths reported during the 2009-2010 season, which included pediatric deaths occurring during the 2009 H1N1 pandemic.
Rates of influenza-associated hospitalizations
During the 2011-2012 season, the highest rates of hospitalizations were in people 65 years and older (30 per 100,000) and in children 0-4 years old (14 per 100,000). The overall rate for all ages was lower in 2011-2012 (8.6 per 100,000) than in 2010-2011 season (21.3 per 100,000); age-group specific rates were also lower in 2011-2012 than in the previous year.
Percentage of visits to outpatient clinics for influenza-like illness
The graph below compares ILI from five different seasons, including the 2011-2012 season, the 2009 H1N1 pandemic season, a ‘moderately severe’ flu season (2007-2008), and a ‘moderate’ season (2002-03).
For a more detailed view of the graphic, please click on the image or visit ILI Weekly National Summary detail.
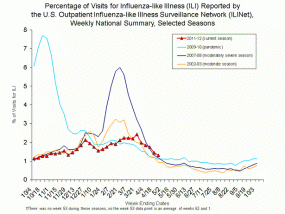
How is severity characterized?
The overall health impact (e.g., illnesses, hospitalizations and deaths) of a flu season varies from year to year. Based on available data from U.S. influenza surveillance systems monitored and reported by CDC, the severity of a flu season can be judged according to a variety of criteria, including:
- The proportion of all deaths that are caused by pneumonia and flu;
- The number of flu-associated deaths among children;
- The proportion of visits to physicians for ILI;
- The flu-associated hospitalization rate among children and adults; and
- The number and proportion of flu laboratory tests that are positive.
A season’s severity is determined by assessing several of these measures and by comparing them with previous seasons.
What flu viruses circulated this season?
During the 2011-2012 season, influenza A (H3N2), 2009 influenza A (H1N1), and influenza B viruses co-circulated in the United States. Over the course of the season, predominant viruses varied from region to region and between states, but nationally, influenza A (H3N2) influenza viruses predominated. Most of the viruses tested this season were well matched to the vaccine viruses (the viruses the 2011-2012 seasonal influenza vaccine is designed to protect against).
How effective was the 2011-2012 seasonal flu vaccine?
Final vaccine effectiveness (VE) estimates for the 2011-2012 influenza season found that influenza vaccine was 47% (95% CI, 36% to 56%) effective at preventing medically-attended acute respiratory illnesses caused by circulating influenza A and B viruses in people of all ages. This estimate is adjusted for age, sex, race/ethnicity, study site, illness onset date, calendar date, assessments of self-rated health and pre-existing medical conditions. These results show moderate effectiveness against circulating influenza viruses, and are similar to the preliminary VE estimate reported earlier in the 2011-2012 season (52%, 95% CI, 38% to 63%). A more detailed report of the final 2011-2012 flu season VE estimates is available in an article published in Clinical Infectious Diseases.
More information about vaccine effectiveness is available at Vaccine Effectiveness – How well does the flu vaccine work? and Flu Vaccine Effectiveness: Questions and Answers for Health Professionals.
Can VE be higher for flu vaccine? Why is flu vaccine recommended when VE for the 2011-2012 season vaccine was about 50%?
The current influenza vaccine is recommended because it is the best way to prevent influenza infections. The substantial burden of influenza-associated illness and death in the United States, combined with overall evidence from a variety of studies, has shown that influenza vaccines do offer protection against influenza illness even when VE is about 50%. Efforts are underway to improve influenza vaccines. The public health community and the federal government are investing in and supporting the development of new and improved influenza vaccines. (For more information on new vaccine research initiatives, contact HHS Biomedical Advanced Research and Development Authority (BARDA). In the meantime, influenza vaccine is the best tool we have to protect as many people as possible against influenza.
What factors influence flu vaccine effectiveness?
How well the flu vaccine works (or its ability to prevent influenza illness) can range widely from season to season and also can vary depending on who is being vaccinated. At least two factors play an important role in determining the likelihood that influenza vaccine will protect a person from influenza illness: 1) characteristics of the person being vaccinated (such as their age and health), and 2) the similarity or “match” between the influenza viruses in the vaccine and those spreading in the community. During years when the viruses in the vaccine and circulating viruses are not well matched, it’s possible that no benefit from vaccination may be observed. During years when the viruses in the vaccine and circulating viruses are very well matched, it’s possible to measure substantial benefits from vaccination in terms of preventing influenza illness. However, even during years when the vaccine match is very good, the benefits of vaccination will vary across the population, depending on characteristics of the person being vaccinated and even, potentially, which vaccine was used.
Researchers try to determine how well a vaccine works in order to continually assess and confirm the value of influenza vaccines as a public health intervention. Study results about how well a flu vaccine works can vary based on study design, outcome(s) measured, population studied and the season in which the vaccine was studied. These differences can make it difficult to compare one study’s results with another’s.
While determining how well a flu vaccine works is challenging, in general, recent studies have supported the conclusion that influenza vaccination benefits public health, especially when the viruses in the vaccine and circulating viruses are well-matched.
What did CDC do to monitor effectiveness of flu vaccines for the 2011-2012 season?
Scientists continued to work on better ways to design, conduct and evaluate non-randomized (i.e., observational) studies to assess how well flu vaccines work. CDC has been working with researchers at universities and hospitals since the 2003-2004 influenza season to estimate how well influenza vaccine works through observational studies using laboratory-confirmed influenza as the outcome. These studies currently use laboratory-confirmed medically-attended influenza virus infections as a specific outcome. CDC’s studies are conducted in five sites across the United States to gather more representative data. To assess how well the vaccine works across different age groups, CDC’s studies of vaccine effects have included all people aged 6 months and older recommended at that time for an annual influenza vaccination. Similar studies are being done in Australia, Canada and Europe.
What did CDC do to monitor antiviral resistance in the United States during the 2011-12 season?
Antiviral resistance means that a virus has changed in such a way that antiviral drugs have become less effective in treating or preventing illnesses caused by the virus. Samples of viruses collected from around the United States and the world are studied to determine if they are resistant to any of the four Food and Drug Administration-approved influenza antiviral drugs.
CDC routinely collects viruses through a domestic and global surveillance system to monitor for changes in influenza viruses. CDC conducted surveillance and testing of influenza viruses to check for antiviral resistance. CDC also worked with the state public health departments and the World Health Organization to collect additional information on antiviral resistance in the United States and worldwide. The information collected assisted in making informed public health policy recommendations.
By the end of the 2011-2012 season, almost all (98.6%) of the 2009 H1N1 influenza viruses tested for antiviral resistance at CDC were susceptible to oseltamivir (Tamiflu®), and all 2009 H1N1 influenza viruses tested were susceptible to zanamivir (Relenza ®). One hundred percent of the H3N2 viruses tested were susceptible to oseltamivir and zanamivir. All of the influenza B viruses tested were susceptible to oseltamivir and zanamivir.
High levels of resistance to the adamantanes (amantadine and rimantadine) persisted among pH1N1 and influenza A (H3N2) viruses currently circulating globally.
Were infections with unusual influenza viruses detected in 2011-2012?
Yes. Thirteen cases of human infection with a novel influenza A (H3N2) variant (H3N2v) virus were reported to CDC. These H3N2v viruses had the M gene from the pH1N1 virus, and are associated with exposure to swine. The thirteen cases were identified in six states: Indiana (two cases), Iowa (three), Maine (two), Pennsylvania (three), Utah (one), and West Virginia (two). One of the 13 cases occurred in an adult, and 12 occurred in children. Three cases resulted in hospitalization; all three patients have recovered fully from their illness. Six of the 13 cases were in persons who reported no recent exposure to swine. In addition, two other novel viruses were identified during the 2011–12 season: one case of influenza A (H1N2) variant (H1N2v) was identified in Minnesota, and one case of influenza A (H1N1) variant (H1N1v) was identified in Wisconsin. One case was in a person who reported close contact with swine preceding symptom onset; both patients are fully recovered.
Since July 2012, there have been outbreaks of H3N2 variant viruses with the matrix (M) gene from the 2009 H1N1 pandemic virus in multiple U.S. states. See Influenza (A) Variant Virus Outbreaks for more information.
- Flu Vaccination Coverage Estimates for the 2011-2012 Flu Season
- 2011-2012 Flu Season Draws to a Close Last full FluView posted May 25, 2012 Shows U.S. Influenza Activity at Summer Levels
- CDC Telebriefing: Influenza Activity Update A transcript of the 2011-2012 Influenza Season Update telebriefing held on Friday, February 23, 2012 at 1PM ET
- Have You Heard, CDC Says U.S. Seeing Latest Flu Season in Nearly Three Decades This HYH summarizes influenza activity to date during 2011-2012 and CDC’s influenza recommendations. Posted Friday, February 10, 2012 5:35:00 PM
- Morbidity and Mortality Weekly Report (MMWR)
Update: Influenza Activity — United States, October 2, 2011–February 11, 2012 This report summarizes U.S. influenza activity since the beginning of the 2011–12 influenza season (October 2, 2011).
