DMI and COVID-19
Through the Data Modernization Initiative, CDC is creating a world in which data can move faster than disease.
The initiative is our response to the challenges public health has been dealing with for too long and that have been highlighted during the COVID-19 pandemic. The pandemic fundamentally changed expectations for public health, especially the speed at which we deliver credible health information. It created an “all hands-on deck” moment to transform how we collect, use, and share data at CDC and beyond.
- Discover how states utilized DMI funding and COVID-19 innovations to advance public health well beyond the pandemic in the CSTE Stories from the Field.
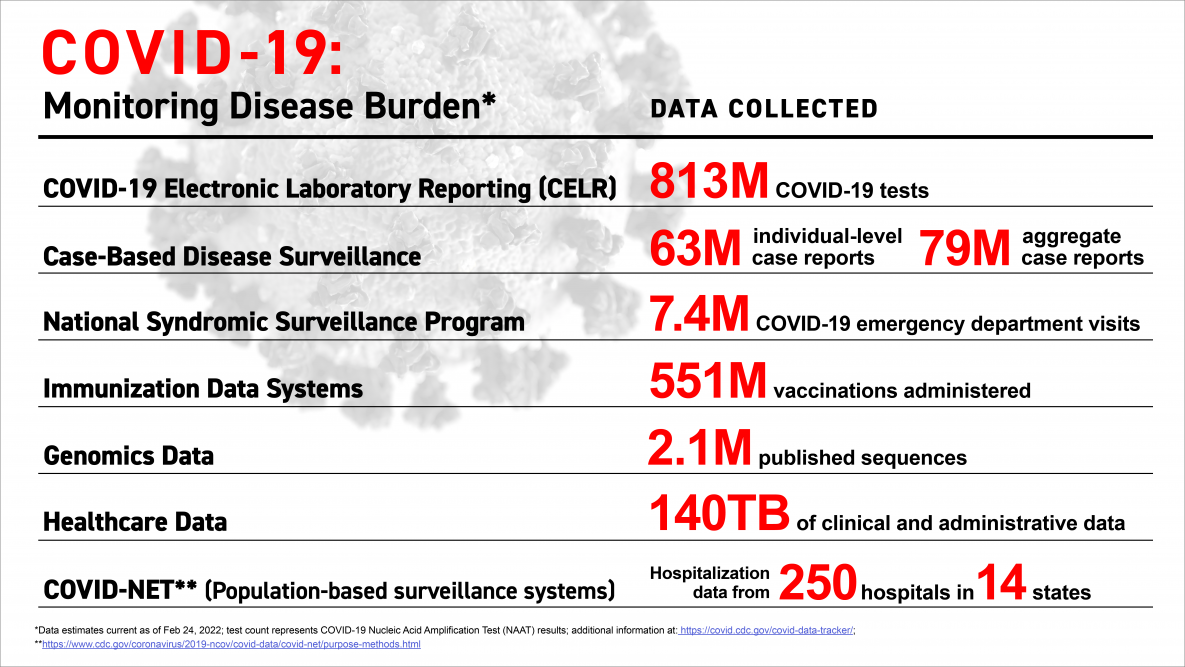
Over the course of the pandemic, CDC has been improving the timeliness, completeness, and quality of critical data for the response.
Progress on modernizing core surveillance systems is helping deliver real-time information through CDC’s COVID Tracker, our “one-stop shop” for COVID-related information. In the first two years of the response, modernization resulted in an unparalleled generation and release of public health data to monitor the burden of COVID-19.
Because of data modernization, we are already in a very different place than we were before the pandemic.
The gains made during the COVID-19 pandemic are now building a bridge to a new kind of surveillance and better approaches to public health data. The examples below demonstrate the power and value of CDC’s Data Modernization Initiative for public health.
Strengthening our nation’s core data sources
Unlocking a new pathway for laboratory results
When the pandemic struck, there was no capability for jurisdictions to report lab data directly to CDC and no national system for tracking both positive and negative test results. In Spring 2020, facing an increasing volume and velocity of testing, CDC stood up COVID Electronic Laboratory Reporting (CELR) to open up a new pathway for laboratory data to get to CDC. Built at breakneck speed, CELR made both positive and negative results available for the first time on any disease.
Massive scale-up of electronic case reporting reaches all states
Electronic case reporting (eCR) offers earlier disease detection and intervention as well as richer, more useful data to drive decisions. CDC massively expanded the nationwide use of eCR. All 50 states, Washington D.C., Puerto Rico, and 12 large jurisdictions can currently receive COVID-19 electronic case reports, up from 21 prior to the pandemic.
Tracking more emergency visits to detect potential outbreaks
Emergency department visits have been used from early on in the pandemic as a leading indicator for community transmission of SARS-CoV-2. Modernization efforts have increased the number of emergency departments participating in syndromic surveillance, as well as the use of these data by public health partners. Recent innovations have also improved the completeness of race information in these reports, which has been critically important for understanding disparities.
Reducing the burden on states for reporting notifiable diseases
At the start of the pandemic, CDC was able to onboard a cohort of states into the national notifiable disease surveillance system in half the usual time. Early data from these efforts allowed CDC’s disease experts and responders in the Emergency Operations Center to better understand and support the national response to COVID-19.
Moving toward real-time reporting of death data
Modernization has allowed CDC to new data on excess deaths and reporting of deaths and life expectancy by race and Hispanic origin. CDC has also shortened the time to release preliminary mortality estimates from months to just weeks. For the first time in 2021, these estimates were released on CDC WONDER.
Unlocking data with cloud-based capabilities
Cloud-based solutions have produced immediate and dramatic results
A key to the COVID-19 response and CDC’s future is having accessible, scalable analytic and visualization capabilities. To advance public health science, CDC stood up a suite of cloud-based services to collect, organize, and connect data across the agency. This enterprise data, analytics, and visualization platform, called the “EDAV,” offers the agency a streamlined way to process data, store it, and visualize it. It provides the means for breaking down data silos and allows CDC scientists to catalogue, analyze and publish findings faster than previously possible.
Cloud capabilities handle millions of genomic sequences
Genomic sequencing is critical to monitoring changes in virus variants and informing allocation decisions on monoclonal therapy. Thanks to new cloud infrastructure and computational capabilities built through data modernization investments, millions of sequences of unique SARS-CoV-2 genomes are now easily handled.
More detailed immunization data is reaching the public faster
To handle the massive COVID-19 vaccine roll-out and delivery in a way that would support effective decision-making, CDC built an Immunization Data Lake capable of receiving and storing many types of data at any scale and increasing analytic capabilities for public health action. Through this solution, CDC has been able to expand vaccine effectiveness data and push that data out to the public faster through the COVID Tracker.
Moving toward real-time, scalable emergency response
Creating a “common operating picture” for real-time, integrated data
Using capabilities put in place through data modernization, CDC developed a cloud-based “common operating picture” for responding to COVID across the nation. The CDC COVID Data Tracker supplies an integrated view on the current state of the American health care system for researchers, policymakers, and the public, including data visualizations on hospitalizations, testing, therapeutics and more.
Understanding COVID-19 vaccine effectiveness
During the initial phases of vaccine rollout in states, only breakthrough cases in vaccinated people were being reported. To better understand how COVID-19 vaccines are working, CDC now monitors rates of COVID-19 cases, hospitalizations, and deaths in both vaccinated and unvaccinated people and is finding automated ways to improve the quality of the data.
New systems to broaden pandemic detection and response
CDC is expanding its platform for multiple respiratory illness surveillance in ways that will better prepare us for the next pandemic or big outbreak – including from diseases like flu, measles, mumps and Legionnaires’ Disease. The System for Outbreak Response, Coordination, and Surveillance (SOURCE) will help detect and manage any size of outbreak, while making data easier to share with state and local partners.
Pandemic-ready solutions for health departments
COVID-19 has highlighted the need for timely, accurate, and automated data that can be used for public health action and emergency response. CDC and the U.S. Digital Service (USDS) have a new multi-year collaboration, including reimagining the data flow, creating pandemic-ready tools, modernizing the hiring process, and implementing discovery sprints.
- As of May 11, 2023, the federal COVID-19 public health emergency has ended, ending CDC’s authorization to collect certain types of public health data.