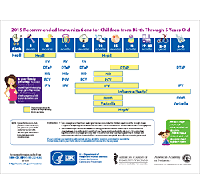
Measles, Mumps, Rubella, Varicella Vaccines
Measles, Mumps, Rubella, and Varicella Diseases and How to Protect Against Them
Measles causes fever, rash, cough, runny nose, and red, watery eyes. Complications can include ear infection, diarrhea, pneumonia, brain damage, and death.
Mumps causes fever, headache, muscle aches, tiredness, loss of appetite, and swollen salivary glands. Complications can include swelling of the testicles or ovaries, deafness, inflammation of the brain and/or tissue covering the brain and spinal cord (encephalitis/meningitis), and, rarely, death.
Rubella causes fever, sore throat, rash, headache, and red, itchy eyes. If a woman gets rubella while she is pregnant, she could have a miscarriage or her baby could be born with serious birth defects.
Varicella (chickenpox) causes blister-like rash, itching, fever, and tiredness. Complications can include severe skin infection, scars, pneumonia, brain damage, or death.
You can protect against these diseases with safe, effective vaccination.
MMRV Vaccine Side Effects
The MMRV vaccine is very safe, and it is effective at preventing measles, mumps, rubella, and varicella. Vaccines, like any medicine, can have side effects. Most people who get MMRV vaccine do not have any serious problems with it. Getting MMRV vaccine is much safer than getting measles, mumps, rubella, or varicella.
- Sore arm from the shot
- Fever
- Mild rash
Some children who get MMRV vaccine may have a seizure caused by fever (febrile seizure) after getting the shot. However, these seizures are not common and have not been associated with any long-term problems.
Rarely, the MMRV vaccine can cause swelling of neck or cheeks or a temporary low platelet count.
Extremely rarely, the vaccine’s ingredients cause severe allergic (anaphylactic) reactions. Children should not get MMRV vaccine if they have ever had a life-threatening allergic reaction to any component of the vaccine, including gelatin or the antibiotic neomycin.
Available MMRV Vaccine
There is one MMRV vaccine approved for use in the United States.
- ProQuad [PDF – 25 pages]: The Food and Drug Administration (FDA) approved this vaccine in 2005 for use in children ages 1 through 12 years of age.
CDC and FDA monitor the safety of vaccines after they are approved or authorized. If a problem is found with a vaccine, CDC and FDA will inform health officials, health care providers, and the public.
CDC uses 3 systems to monitor vaccine safety:
- The Vaccine Adverse Event Reporting System (VAERS): an early warning system, co-managed by CDC and FDA, to monitor for potential vaccine safety problems. Anyone can report possible vaccine side effects to VAERS.
- The Vaccine Safety Datalink (VSD): a collaboration between CDC and 13 healthcare organizations that conducts vaccine safety monitoring and research.
- The Clinical Immunization Safety Assessment (CISA) Project: a partnership between CDC and several medical research centers that provides expert consultation and conducts clinical research on vaccine-associated health risks.
A Closer Look at the Safety Data
- Before the MMRV vaccine was licensed for use in the United States, researchers studied the vaccine in children aged 12 to 23 months old. The studies found that rash and a fever of 102°F or higher happened more often during the 42 days after the first dose of MMRV vaccine compared with separate injections of MMR and varicella vaccines. There is no increased risk of febrile seizures after vaccination with MMRV vaccine in children aged 4 through 6 years. Febrile seizures have not been associated with any long-term effects.
- Soreness from the shot was reported less often after MMRV vaccine than after MMR and varicella vaccines given in separate shots at the same visit. For more information, see “Results from Studies Before MMRV Vaccine Was Licensed.”
More Resources
- MMRV Vaccine Information Statement
- MMRV Vaccine: Who Should Not Get Vaccinated
- Two Options for Protecting Your Child Against Measles, Mumps, Rubella, and Varicella
- Measles, Mumps, and Rubella Vaccine Safety
- Varicella Vaccine Safety
- Q&As About the Options for Protecting Your Child Against Measles, Mumps, Rubella, and Varicella
- Fact Sheet for Healthcare Providers: MMR & Varicella Vaccines or MMRV Vaccine – Discussing Options with Parents
- Q&As About Vaccination Options for Preventing Measles, Mumps, Rubella, and Varicella: Questions and Answers for Healthcare Providers
Related Scientific Articles
CDC. Use of Combination Measles, Mumps, Rubella, and Varicella Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2010 May 07;59(RR03):1-12.
Hornig M, Briese T, Buie T, Bauman ML, Lauwers G, et al. Lack of association between measles virus vaccine and autism with enteropathy: A case-control study.External PLoS One. 2008 Sep 4;3(9):e3140.
Klein NP, Fireman B, Yih WK, Lewis E, Kulldorff M. Measles-mumps-rubella-varicella combination vaccine and the risk of febrile seizuresExternal. Pediatrics. 2010 Jul;126(1):e1-8.
Klein NP, Lewis E, Baxter R, et al. Measles-containing vaccines and febrile seizures in children age 4 to 6 yearsExternal. Pediatrics. 2012 May;129(5):809 -814.
Rowhani-Rahbar A, Fireman B, Lewis E, Nordin J, Naleway A, et al. Effect of age on the risk of Fever and seizures following immunization with measles-containing vaccines in childrenExternal. JAMA Pediatr. 2013 Dec;167(12):1111-7.