Recommendations for Fully Vaccinated People
COVID-19 Homepage

Easy as 1-2-3
Interpretive Summary for August 27, 2021
Easy as 1-2-3
On August 23, 2021, the U.S. Food and Drug Administration (FDA) granted full approvalExternal of the Comirnaty/Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 disease in people ages 16 years and older. The vaccine continues to be available under emergency use authorization for adolescents ages 12 to 15 years, and for the administration of a third dose in some immunocompromised people. As of August 26, 2021, 203 million people in the United States have received at least one dose of a COVID-19 vaccine. 172 million people, or about 52% of the U.S. population, are fully vaccinated.
Studies show some immunocompromised people don’t always build the same level of immunity after vaccination the way non-immunocompromised people do. When this happens, getting another dose of the vaccine can sometimes help them build more protection against the disease. CDC recommends that people with moderately to severely compromised immune systems receive an additional (third) dose of a COVID-19 mRNA (Pfizer-BioNTech or Moderna) vaccine after the initial 2-doses. People who are moderately to severely immunocompromised are at increased risk of serious, prolonged illness due to COVID-19. In small studies Pdf[36 pages, 2 MB], fully vaccinated immunocompromised people have made up a large proportion of hospitalized “breakthrough infections.”
On August 23, 2021, the U.S. Food and Drug Administration (FDA) granted full approvalExternal of the Comirnaty/Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 disease in people ages 16 years and older. The vaccine continues to be available under emergency use authorization for adolescents ages 12 to 15 years, and for the administration of a third dose in some immunocompromised people. As of August 26, 2021, 203 million people in the United States have received at least one dose of a COVID-19 vaccine. 172 million people, or about 52% of the U.S. population, are fully vaccinated.
Studies show some immunocompromised people don’t always build the same level of immunity after vaccination the way non-immunocompromised people do. When this happens, getting another dose of the vaccine can sometimes help them build more protection against the disease. CDC recommends that people with moderately to severely compromised immune systems receive an additional (third) dose of a COVID-19 mRNA (Pfizer-BioNTech or Moderna) vaccine after the initial 2-doses. People who are moderately to severely immunocompromised are at increased risk of serious, prolonged illness due to COVID-19. In small studiesPdf, fully vaccinated immunocompromised people have made up a large proportion of hospitalized “breakthrough infections.”
A new CDC study shows the COVID-19 vaccines authorized in the United States continue to be highly effective at reducing the risk of severe COVID-19 illness, hospitalization, and death. However, recent data show that protection against asymptomatic, mild, and moderate disease may decrease over time. This does not mean that COVID-19 vaccines are no longer working. The reduced protection may be due to both decreasing immunity over time and the highly contagious Delta variant. On August 18, 2021, U.S. Health and Human Services public health and medical experts announced a plan to begin offering COVID-19 mRNA vaccine booster shots this fall. Booster shots are doses of vaccine that are given when the initial immune response to a vaccine is likely to have decreased over time.
COVID-19 vaccines remain the most powerful tool we have against COVID-19, making it critical that all people get vaccinated as soon as they are eligible. To find a vaccine provider near you, visit vaccines.gov or your state or local public health department. If you are immunocompromised and have questions, talk with your healthcare professional about your medical condition and whether an additional dose of a COVID-19 vaccine is right for you.
Note to readers: COVID-19 vaccine boosters may be available starting this fall with people being eligible starting 8 months after they received their second dose of an mRNA vaccine (either Pfizer-BioNTech or Moderna). This is subject to authorization by the FDA and recommendation by CDC’s Advisory Committee on Immunization Practices (ACIP). It is likely that people who received a Johnson & Johnson/Janssen (J&J) COVID-19 vaccine will also need a booster dose. Because the J&J vaccine wasn’t given in the United States until 70 days after the first mRNA vaccine doses, the data are not yet available but are expected in the coming weeks. With those data in hand, CDC will keep the public informed with a timely plan for J&J booster shots.
Reported Cases
The current 7-day moving average of daily new cases (142,006) increased 2.8% compared with the previous 7-day moving average (138,087). The current 7-day moving average is 107.2% higher compared to the peak observed on July 20, 2020 (68,522). The current 7-day moving average is 44.1% lower than the peak observed on January 10, 2021 (253,932) and is 1,117.9% higher than the lowest value observed on June 18, 2021 (11,660). A total of 38,341,339 COVID-19 cases have been reported as of August 25, 2021.
38,341,339
Total Cases Reported
38,341,339
Total Cases Reported
142,006
Current 7-Day Average*
142,006
Current 7-Day Average*
138,087
Prior 7-Day Average
138,087
Prior 7-Day Average
+2.8%
Change in 7-Day Average since Prior Week
+2.8%
Change in 7-Day Average since Prior Week
*Historical cases are excluded from daily new cases and 7-day average calculations until they are incorporated into the dataset for the applicable date. Of 104,743 historical cases reported retroactively, 10,269 were reported in the current week and 3,546 were reported in the prior week.
Daily Trends in COVID-19 Cases in the United States Reported to CDC

7-Day moving average
SARS-CoV-2 Variants
Multiple variants of the virus that causes COVID-19 are circulating globally, including within the United States. Currently, four variants are classified as a variant of concern (VOC). Nowcast estimates* of COVID-19 cases caused by these VOCs for the week ending August 21, 2021, are summarized here. Nationally, the combined proportion of cases attributed to Delta is estimated to be greater than 99%; Alpha proportion is estimated to decrease to 0.2%; Gamma proportion is estimated to decrease to 0.1%; and Beta is estimated to be less than 0.1%. Nowcast estimates that Delta will continue to be the predominant variant circulating in all 10 U.S. Department of Health and Human Services (HHS) regions. Alpha is estimated to be 0.3% or less in all HHS regions. Gamma is estimated to be 0.3% or less in all HHS regions; and Beta is estimated to be less than 0.1% in all HHS regions.
*The median time from specimen collection to sequence data reporting is about 3 weeks. As a result, weighted estimates for the most recent few weeks may be unstable or unavailable. CDC’s Nowcast is a data projection tool that helps fill this gap by generating timely estimates of variant proportions for variants that are circulating in the United States. View Nowcast estimates on CDC’s COVID Data Tracker website on the Variant Proportions page.
Testing
The percentage of COVID-19 NAATs (nucleic acid amplification tests)* that are positive (percent positivityExternal) has decreased from the previous week. The 7-day average of percent positivity from tests is now 10.1%. The 7-day average number of tests reported for August 13–August 19, 2021, was 1,313,164, up 9.8% from 1,195,677 for the prior 7 days.
518,701,870
Total Tests Reported
518,701,870
Total Tests Reported
1,313,164
7-Day Average Tests Reported
1,313,164
7-Day Average Tests Reported
10.1%
7-Day Average % Positivity
10.1%
7-Day Average % Positivity
10.4%
Previous 7-Day Average % Positivity
10.4%
Previous 7-Day Average % Positivity
-3.1%
Change in 7-Day Average % Positivity since Prior Week
-3.1%
Change in 7-Day Average % Positivity since Prior Week
*Test for SARS-CoV-2, the virus that causes COVID-19
COVID-19 NAAT Laboratory Test 7-day Percent Positivity by State/Territory
Vaccinations
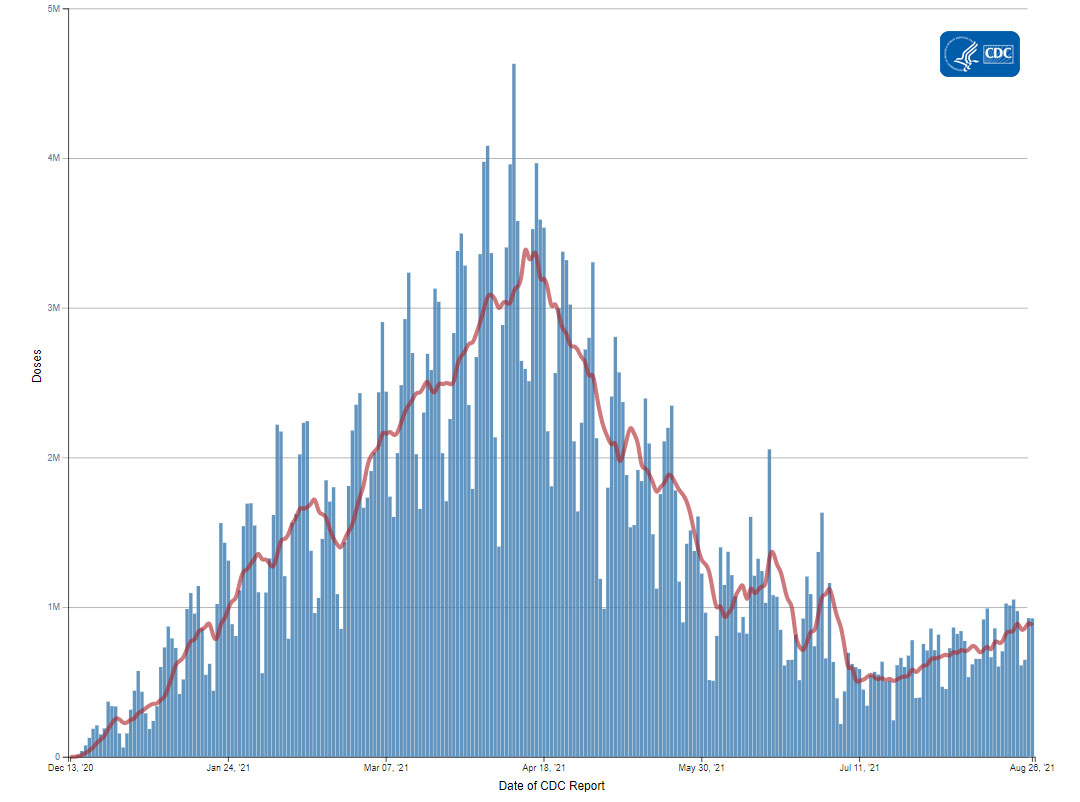
The U.S. COVID-19 Vaccination Program began December 14, 2020. As of August 26, 2021, 365.8 million vaccine doses have been administered. Overall, about 203 million people, or 61.1% of the total U.S. population, have received at least one dose of vaccine. About 172.2 million people, or 51.9% of the total U.S. population, have been fully vaccinated.* As of August 26, the 7-day average number of administered vaccine doses reported (by date of CDC report) to CDC per day was 877,756, a 6.61% increase from the previous week.
CDC’s COVID Data Tracker Vaccination Demographic Trends tab shows vaccination trends by age group. As of August 26, 2021, 91.7% of people ages 65 years or older have received at least one dose of vaccine and 81.4% are fully vaccinated. Over two-thirds (73.5%) of people ages 18 years or older have received at least one dose of vaccine and 62.8% are fully vaccinated. For people ages 12 years or older, 71.5% have received at least one dose of vaccine and 60.7% are fully vaccinated.
365,767,674
Vaccines Administered
365,767,674
Vaccines Administered
202,961,676
People who received at least one dose
202,961,676
People who received at least one dose
172,171,009
People who are fully vaccinated*
172,171,009
People who are fully vaccinated*
61.1%
Percentage of the US population that has received at least one dose
61.1%
Percentage of the US population that has received at least one dose
51.9%
Percentage of the US population that has been fully vaccinated*
51.9%
Percentage of the US population that has been fully vaccinated*
+0.9
Percentage point increase from last week
+0.9
Percentage point increase from last week
+0.8
Percentage point increase from last week
+0.8
Percentage point increase from last week
*Represents the number of people who have received the second dose in a two-dose COVID-19 vaccine series (such as the Pfizer or Moderna vaccines),) or one dose of the single-shot Johnson & Johnson’s Janssen vaccine.
Daily Change in the Total Number of Administered Doses Reported to CDC by the date of CDC Report, United States

7-Day moving average
Hospitalizations
New Hospital Admissions
The current 7-day daily average for August 18–August 24, 2021 was 12,297. This is a 5.7% increase from the prior 7-day average (11,637) from August 11–August 17, 2021. Nationally, new admissions of patients with confirmed COVID-19 are currently at their highest levels since the start of the pandemic for all age groups under 50 years old.
New admissions of patients with confirmed COVID-19 are currently at their highest levels since the start of the pandemic in Hawaii, Kentucky, Oregon, Washington, and Wyoming.
2,674,920
Total New Admissions
2,674,920
Total New Admissions
12,297
Current 7-Day Average
12,297
Current 7-Day Average
11,637
Prior 7-Day Average
11,637
Prior 7-Day Average
+5.7%
Change in 7-Day Average
+5.7%
Change in 7-Day Average
The start of consistent reporting of hospital admissions data was August 1, 2020.
Daily Trends in Number of New COVID-19 Hospital Admissions in the United States
New admissions are pulled from a 10 am EST snapshot of the HHS Unified Hospital Timeseries Dataset. Due to potential reporting delays, data from the most recent 7 days, as noted in the figure above with the grey bar, should be interpreted with caution. Small shifts in historic data may also occur due to changes in the Centers for Medicare and Medicaid Services (CMS) Provider of Services file, which is used to identify the cohort of included hospitals.
COVID-NET: Trends in Rates of Hospitalizations in Adults Ages 18 Years and Older
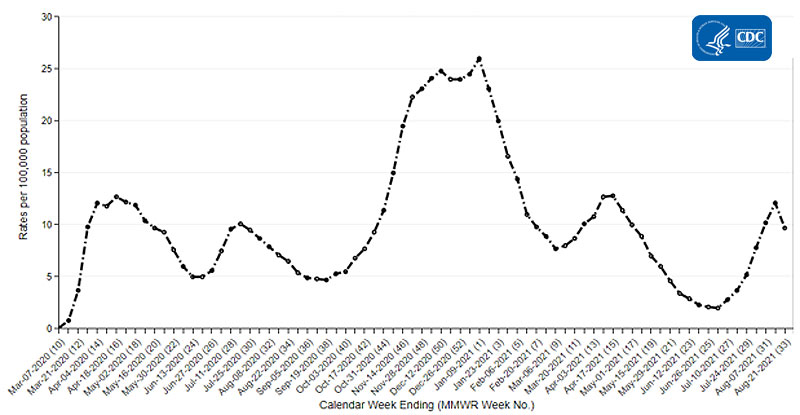
CDC’s Coronavirus Disease 2019-Associated Hospitalization Surveillance Network (COVID-NET) shows that rates of COVID-19-associated hospitalizations are increasing for people of all ages. Rates of COVID-19-associated hospitalizations in adults 18 years and older have steadily increased over recent weeks, from 2.0 hospitalizations per 100,000 population for the week ending July 3, 2021, to 10.2 per 100,000 for the week ending August 7, 2021, a dramatic increase over a five-week period.
Trends in Rates of Hospitalizations in Adults Ages 18 Years and Older
The Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET) is an additional source for hospitalization data collected through a network of more than 250 acute-care hospitals in 14 states (representing ~10% of the U.S. population). Detailed data on patient demographics, including race/ethnicity, underlying medical conditions, medical interventions, and clinical outcomes, are standardized case reporting form.
Deaths
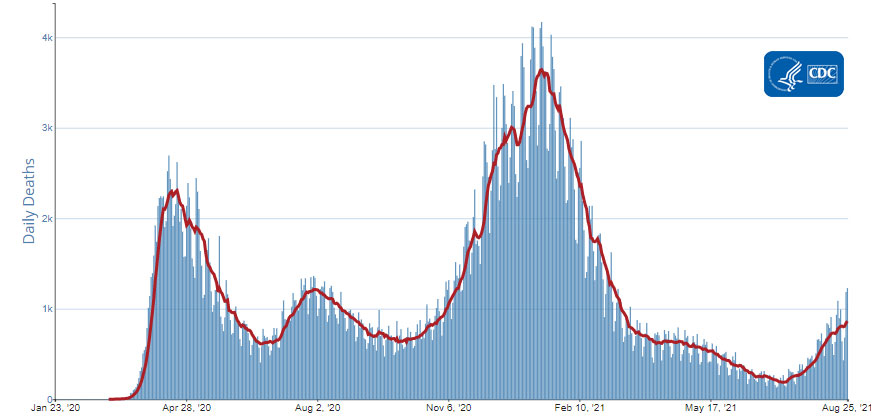
The current 7-day moving average of new deaths (864) has increased 11.0% compared with the previous 7-day moving average (779). The current 7-day moving average is 28.8% lower compared to the peak observed on July 31, 2020 (1,214). The current 7-day moving average is 76.3% lower than the peak observed on January 13, 2021 (3,643) and is 354.5% higher than the lowest value observed on July 10, 2021 (190). As of August 25, 2021, a total of 631,440 COVID-19 deaths have been reported in the United States.
631,440
Total Deaths Reported
631,440
Total Deaths Reported
864
Current 7-Day Average*
864
Current 7-Day Average*
779
Prior 7-Day Average
779
Prior 7-Day Average
+11.0%
Change in 7-Day Average Since Prior Week
+11.0%
Change in 7-Day Average Since Prior Week
*Historical deaths are excluded from the daily new deaths and 7-day average calculations until they are incorporated into the dataset by their applicable date. Of 6,955 historical deaths reported retroactively, 181 were reported in the current week and 37 were reported in the prior week.
Daily Trends in Number of COVID-19 Deaths in the United States Reported to CDC

7-Day moving average
- Effectiveness of COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Frontline Workers Before and During B.1.617.2 (Delta) Variant Predominance — Eight U.S. Locations, December 2020–August 2021
- SARS-CoV-2 Infections and Hospitalizations Among Persons Aged ≥16 Years, by Vaccination Status — Los Angeles County, California, May 1–July 25, 2021
- Mental Health and Substance Use Among Adults with Disabilities During the COVID-19 Pandemic — United States, February–March 2021
- New COVID-19 Cases and Hospitalizations Among Adults, by Vaccination Status — New York, May 3–July 25, 2021
- Sustained Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Associated Hospitalizations Among Adults — United States, March–July 2021
- Effectiveness of Pfizer-BioNTech and Moderna Vaccines in Preventing SARS-CoV-2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant — National Healthcare Safety Network, March 1–August 1, 2021
Recent COVID Data Tracker Updates
- The Vaccinations in the US tab now includes data on additional dose administration