Recommendations for Fully Vaccinated People
COVID-19 Homepage
Understanding How COVID-19 Vaccines Work
What You Need to Know
- COVID-19 vaccines help our bodies develop immunity to the virus that causes COVID-19 without us having to get the illness.
- Different COVID-19 vaccines may work in our bodies differently but all provide protection against the virus that causes COVID-19.
- None of the COVID-19 vaccines can give you COVID-19.
- Bringing new vaccines to the public involves various steps, all which must be followed to ensure they are safe and effective before they are made available for use.
How COVID-19 Vaccines Work
COVID-19 vaccines help our bodies develop immunity to the virus that causes COVID-19 without us having to get the illness.

Different types of vaccines work in different ways to offer protection. But with all types of vaccines, the body is left with a supply of “memory” T-lymphocytes as well as B-lymphocytes that will remember how to fight that virus in the future.
It typically takes a few weeks after vaccination for the body to produce T-lymphocytes and B-lymphocytes. Therefore, it is possible that a person could be infected with the virus that causes COVID-19 just before or just after vaccination and then get sick because the vaccine did not have enough time to provide protection.
Sometimes after vaccination, the process of building immunity can cause symptoms, such as fever. These symptoms are normal signs the body is building immunity.
Types of Vaccines: mRNA, Viral Vector, and Protein Subunit
There are different types of vaccines.
- All COVID-19 vaccines prompt our bodies to recognize and help protect us from the virus that causes COVID-19.
- Currently, there are three types of COVID-19 vaccines for use in the United States: mRNA, protein subunit, and viral vector vaccines.
- As of May 6, 2023, J&J/Janssen COVID-19 vaccine, a viral vector vaccine, has expired and is no longer available for use in the United States.
None of these vaccines can give you COVID-19.
- Vaccines do not use any live virus.
- Vaccines cannot cause infection with the virus that causes COVID-19 or other viruses.
They do not affect or interact with our DNA.
- These vaccines do not enter the nucleus of the cell where our DNA (genetic material) is located, so it cannot change or influence our genes.
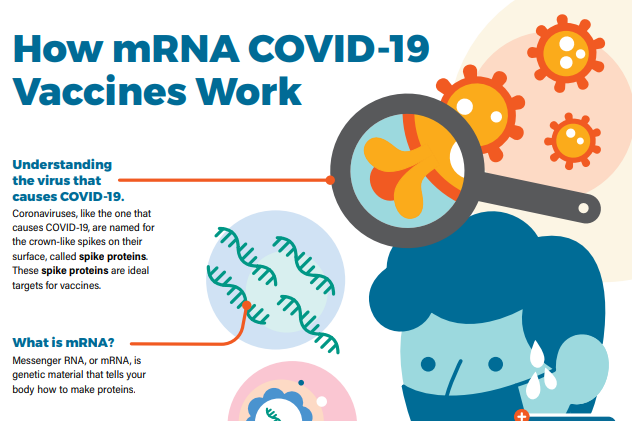
mRNA vaccines (Pfizer-BioNTech or Moderna)
To trigger an immune response, many vaccines put a weakened or inactivated germ into our bodies. Not mRNA vaccines. Instead, mRNA vaccines use mRNA created in a laboratory to teach our cells how to make a protein—or even just a piece of a protein—that triggers an immune response inside our bodies. This immune response, which produces antibodies, is what helps protect us from getting sick from that germ in the future.
About Pfizer-BioNTech and ModernaResearch for mRNA technology
Researchers have been studying and working with mRNA vaccines for decades.
- In fact, mRNA vaccines have been studied before for flu, Zika, rabies, and cytomegalovirus (CMV).
- Beyond vaccines, cancer research has also used mRNA to trigger the immune system to target specific cancer cells.
Protein subunit vaccines (Novavax)
Protein subunit vaccines contain pieces (proteins) of the virus that causes COVID-19. These virus pieces are the spike protein. The vaccine also contains another ingredient called an adjuvant that helps the immune system respond to that spike protein in the future. Once the immune system knows how to respond to the spike protein, the immune system will be able to respond quickly to the actual virus spike protein and protect you against COVID-19.
About NovavaxResearch for protein subunit technology
Protein subunit vaccines have been used for years.
- More than 30 years ago, a hepatitis B vaccine became the first protein subunit vaccine to be approved for use in people in the United States.
- Another example of other protein subunit vaccines used today include whooping cough vaccines.
Viral vector vaccines (Johnson & Johnson’s Janssen)
As of May 6, 2023, J&J/Janssen COVID-19 vaccine has expired and is no longer available for use in the United States.
Viral vector COVID-19 vaccines use a modified version of a different virus (a vector virus) to deliver important instructions to our cells.
About Johnson & Johnson's JanssenResearch for viral vector technology
For decades, hundreds of scientific studies of viral vector vaccines have been done and published around the world.
- Some vaccines recently used for Ebola outbreaks have used viral vector technology.
- Several studies have focused on viral vector vaccines against other diseases such as Zika, flu, and HIV.
- Besides being used in vaccines, viral vectors have also been studied for gene therapy, to treat cancer, and for molecular biology research.
Developing COVID-19 Vaccines
While COVID-19 vaccines were developed rapidly, all steps have been taken to ensure their safety and effectiveness. Bringing a new vaccine to the public involves many steps including:
- vaccine development,
- clinical trials,
- U.S. Food and Drug Administration (FDA) authorization or approval,
- and development and approval of vaccine recommendations through the Advisory Committee on Immunization Practices (ACIP) and CDC.
As vaccines are distributed outside of clinical trials, monitoring systems are used to make sure that COVID-19 vaccines are safe.
- Do you need to wait to get vaccinated after getting COVID-19 or getting treatment for COVID-19?
- How can you prepare for vaccination?
- What can you expect during and after your vaccination?