Understanding EGAPP




| Group | Topics | Evidence Report | Recommendation Statement | Dissemination |
|---|---|---|---|---|
| EGAPP Working Group (EWG) |  |
 |
||
| EGAPP consultants and CDC-based staff |  |
 |
 |
 |
| EWG members who serve on Technical Expert Panel |  |
 |

|
|
| EWG who serve on the recommendation writing team |  |
 |
||
| Stakeholders and interested public |  |
 |
||
| Evidence-based Practice Centers (EPc) and other review groups |  |
 |
||
| Expert and /or peer reviewers |  |
 |
||
| Industry/test developer* invited comment on the final evidence report |
 |
 |

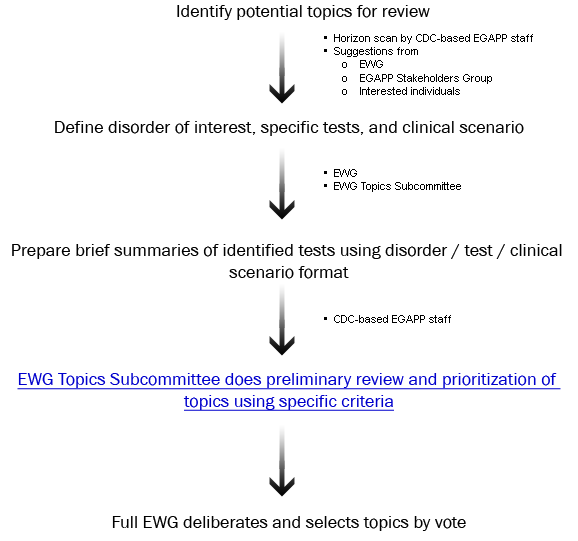

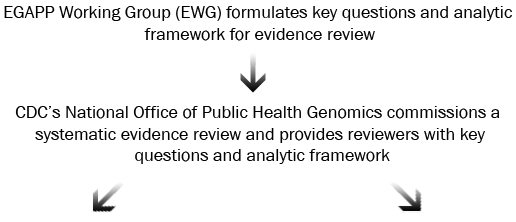
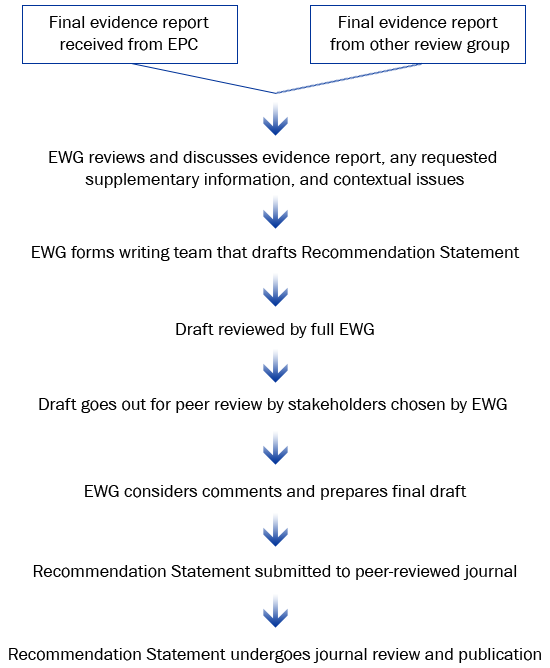
In some cases, the EWG may request additional information, analysis, or targeted review to address questions that arise as part of the development of the Recommendation Statement.



For more information see EGAPP FAQs or CDC EGAPP Initiative.
Primary EGAPP products
Commissioned evidence reports
- Posted on EWG website or Agency for Healthcare Research and Quality website
EWG Recommendation Statements
- Published in peer-reviewed journal
- Free web access to article
- Journal may issue press release
EWG website
- Open access
Secondary EGAPP products
Announcements of EGAPP initiative primary products and other updates (CDC-based EGAPP staff)
- Posted on the website of the CDC Office of Public Health Genomics (OPHG), which supports the EGAPP initiative.
- Posted on the EWG website
- Distributed by e-mail across CDC and to EGAPP stakeholder list (approximately 400 genetic testing stakeholder organizations and individuals)
EGAPP informational messages incorporated into translational materials for providers and consumers (CDC-based EGAPP staff, ESG)
- Posted on CDC OPHG web pages
- Disseminated by various means through diverse EGAPP Stakeholders Group organizations
CDC Press Statements related to EGAPP (CDC-based EGAPP staff and CDC press office)
- Posted on CDC web pages for Media
EGAPP initiative web pages on CDC website (CDC-based EGAPP staff)
- Posted on CDC OPHG web pages
EGAPP initiative posters and presentations (CDC EGAPP staff, EWG, ESG)
View Page In: PDF [1M]