Appendix: Summary of Systematic Review and Meta-analysis of the Effectiveness of LAIV Among Children Aged 2 through 17 Years
Background
Routine annual influenza vaccination of all children ≥6 months of age, regardless of health status, has been recommended in the United States by the Advisory Committee on Immunization Practices (ACIP) and CDC since 2008 (1). Prior to the 2014-15 season, either inactivated influenza vaccine (IIV; for those ≥6 months of age) or live attenuated influenza vaccine (LAIV; for those ≥2 years of age) was recommended for healthy children 2 years of age and older, with no preference for one or the other. Until the 2013-14 season, seasonal (non-pandemic) IIV and LAIV were trivalent vaccines (IIV3 and LAIV3, respectively), containing an influenza A(H1N1) virus, an influenza A(H3N2) virus, and an influenza B virus (representing one of the two influenza B lineages). Quadrivalent influenza vaccines (containing the same viruses as trivalent vaccines, with the addition of a second influenza B virus of the other lineage) became available in the U.S. during the 2013-14 season. From 2013-14 through 2017-18, both trivalent and quadrivalent IIV (IIV3 and IIV4) have been available in the U.S., while all LAIV has been quadrivalent (LAIV4).
In June 2014, following review of data from controlled trials indicating superior efficacy of LAIV3 compared with IIV3 from studies conducted prior to the 2009 pandemic (2, 3), ACIP expressed a preferential recommendation for LAIV3 for healthy children aged 2 through 8 years. Subsequently, analysis of data from U.S. observational studies of the 2013-14 and 2015-16 seasons revealed no statistically significant effectiveness of LAIV4 against influenza A(H1N1)pdm09 among children aged 2 through 17 years (4, 5). In contrast, IIV was effective against influenza A(H1N1)pdm09 during these two seasons. Ongoing review of these data at consecutive ACIP meetings led to the decision in February 2015 that a preference for LAIV was no longer warranted (6), and subsequently to a decision in June 2016 that LAIV should not be used in the United States during the 2016-17 season (7). This recommendation was extended for the 2017-18 season (8).
Objectives
The primary objective of this systematic review was to determine if vaccination with live attenuated influenza vaccine (LAIV) is associated with prevention of laboratory-confirmed influenza illness among children aged 2 through 17 years. The secondary objective was to examine if protection provided by LAIV differs from that provided by inactivated influenza vaccine (IIV) among children aged 2 to 17 years.
Methods
Studies eligible for inclusion evaluated receipt of at least one dose of LAIV3 or LAIV4 compared with placebo, non-influenza control vaccine, inactivated influenza vaccines, or no vaccine among children 2 through 17 years of age during the 2010-11 through 2016-17 influenza seasons. The outcomes of interest were laboratory-confirmed influenza (LCI)-associated outcomes (including laboratory-confirmed medically-attended outpatient influenza infection and laboratory-confirmed influenza-associated hospitalizations), in which laboratory confirmation was obtained by viral culture and/or polymerase chain reaction. The following were excluded: animal studies; studies exclusively of adults aged 18 years and older or children aged 2 years and under; studies involving products (both LAIV and comparators) not licensed for use in the United States (e.g., virosomal vaccines, LAIV constructed on a different backbone from U.S.-licensed LAIV [e.g., Leningrad backbone]); studies restricted to special populations (e.g., immunosuppressed patients), duplicate reports, and interim reports superseded by a final report.
A systematic literature search strategy was developed with an expert librarian. For the initial search, MEDLINE, EMBASE, CINAHL, Scopus, ClinicalTrials.gov, and the Cochrane Library Center Register of Controlled Trials to identify English language papers meeting the above criteria, indexed between January 1, 2011 and December 1, 2016. A subsequent search was performed to identify papers published though October 31, 2017. The search strategies are summarized below.
A minimum of two reviewers independently screened report titles and abstracts for potential inclusion. Papers determined during the screening of titles and abstracts to be potentially eligible for inclusion were assessed independently by a minimum of two reviewers. Differences in assessment for inclusion were resolved through discussion and consensus. Reference lists of eligible articles were reviewed to help identify additional published studies.
Selected articles were independently reviewed by at least two abstractors to collect information on eligibility criteria, study characteristics, and effect measures and their variance. Estimates of both absolute (for studies comparing LAIV with placebo, non-influenza vaccines, or no vaccination) and relative (for studies comparing LAIV with IIV) vaccine effectiveness were abstracted. Abstracted data were discussed among the reviewers and disagreements resolved through consensus. Study authors were contacted in some instances to clarify questions related to methods or results.
Study quality was assessed using ROBINS-I (Risk of Bias in Non-randomized Studies of Interventions) (9). This is a structured instrument which provides a framework for assessment of risk of bias in seven domains (confounding, selection of participants, classification of interventions, deviation from intended intervention, missing data, measurement of outcomes, and selection of reported results. Risk of Bias is reported as Low, Moderate, Serious, or Critical. For the first domain, risk of bias due to confounding, studies were assessed with regard to whether the effect estimates were adjusted for age, geographic site (for multisite studies), season (for multiseason studies), presence of asthma (adjustment for high-risk medical condition accepted as proxy for asthma if asthma included among those conditions), and calendar time of illness onset.
Effect estimates judged to have been derived using sufficiently comparable methods were subject to meta-analysis. Effect estimates were pooled using a random effects model, in which the pooled estimate is a weighted average of the study-specific effect estimates (measured as the log odds ratio). Weight was defined as the inverse of the estimated variance. Where available, adjusted effect estimates were used. Estimates were pooled for all influenza due to any type or subtype, for influenza A(H1N1)pdm09, for influenza A(H3N2), and for influenza B. In cases where no estimate was reported for all influenza types and subtypes combined, a crude estimate was calculated, where possible, from the available type and subtype-specific estimates.
Results
A total of 1,136 articles were retrieved, of which 18 were judged to meet inclusion criteria. These included 2 cluster randomized trials (10, 11) and 16 observational studies (5, 12-26). No individually randomized trials were identified. Given the small number of cluster randomized trials and that the results could not be pooled with the observational studies, as well as the lack of individually randomized studies, the meta-analysis focused on the 16 observational studies.
The characteristics and assessed risk of bias of the observational studies are summarized in Table: Characteristics of observational studies. Ten (63%) were conducted in the United States, 3 in the United Kingdom, 2 in Canada, and 1 in Germany. The majority (15 [93%]) used a test-negative case-control design, in which eligible children presented for outpatient medical care with acute respiratory symptoms, and cases were those who tested positive for influenza by either PCR or culture.
Most (75%) studies were assessed to have moderate risk of bias. A common factor among the studies judged to have serious risk of bias was lack of control for at least one potential confounding domain of interest. Importantly, however, in these studies overall sample sizes and numbers of influenza cases were relatively small, making adjustment for additional parameters inappropriate.
Meta-analysis results are summarized in Figures 1 through 7. In terms of absolute effectiveness as compared with no vaccine, LAIV was effective against influenza due to any type or subtype (VE=45%, 95% CI: 32-56%; Figure 1). LAIV was also effective, though less so, against influenza A(H1N1)pdm09 (VE=25%, 95% CI: 6-40%; Figure 2). When stratifying by location (U.S. vs. non-U.S.), LAIV was significantly effective only among non-U.S. studies (Figure 3). Stratifying by LAIV formulation (LAIV4 vs. LAIV3), LAIV4 was effective against influenza A(H1N1)pdm09 (Figure 4). The pooled effect estimate for LAIV3 was not statistically significant and was imprecise, likely due to the small number of estimates in this stratum.
Considering relative odds for influenza infection among children receiving LAIV compared with those receiving IIV, for influenza A(H1N1)pdm09, IIV was significantly more effective than LAIV against influenza A(H1N1)pdm09 viruses (odds of influenza with LAIV vs. IIV= 2.52, 95% CI: 1.58-4.02; Figure 5). For influenza B viruses, the point estimate of the odds ratio favored LAIV, but the estimate was not statistically significant (odds of influenza with LAIV vs. IIV= 0.55, 95% CI: 0.27-1.12; Figure 6). For influenza A(H3N2) viruses, LAIV and IIV performed similarly (odds of influenza with LAIV vs. IIV= 1.01, 95% CI: 0.73-1.38; Figure 7).
The two cluster randomized trials were conducted in Canada. Both involved comparisons of LAIV3 vs. IIV3. The first was a pilot study designed to assess direct (among vaccinees) and indirect (among household contacts) benefits of LAIV3 vs. IIV3 (10). In this study, 10 Ontario elementary schools were randomized 1:1 to administer either LAIV3 or IIV3. A total of 166 vaccinated students were analyzed (109 of whom received LAIV3 and 57 of whom received IIV3). The majority of PCR-confirmed influenza illnesses detected involved influenza A(H1N1) viruses (22 infections total among students and household members, including 21 influenza A[H1N1] infections and 1 influenza B infection). Among vaccinated students, incidence of influenza was lower among those who received LAIV3 than those who received IIV3 (incidence rate ratio [IRR]=0.10, 95%CI 0.002—0.94). These results contrast with those of the observational studies for the H1N1-predominant 2013-14 season, which generally showed IIV3 was more protective. The second study conducted among 52 Hutterite communities in Alberta and Saskatchewan during the 2012-13, 2013-14, and 2014-15 seasons. In this study, communities were randomized 1:1 to administer either LAIV3 or IIV3 to children aged 3 through 15 years (11). The primary outcome was to compare protection against influenza among all community members afforded by vaccinating children with LAIV3 vs. IIV3. Subanalyses restricted to the vaccinated children were reported as a secondary outcome. No significant difference between LAIV3 and IIV3 was noted for all influenza (A and B viruses) pooled across all three seasons (hazard ratio [HR]=0.97, 95%CI 0.71—1.34); similarly, no significant difference was noted when each year of the study was analyzed individually. For influenza A viruses, with all three seasons pooled, IIV3 offered better protection (HR=1.62, 95%CI 1.02—2.59). In analysis of individual seasons, this was driven primarily by better protection by IIV3 during the H3N2-predominant 2012-13 season (HR=6.70, 95%CI 1.15—39.14). In contrast to this finding, no significant difference between LAIV3 and IIV3 was noted for the H3N2-predominant 2014-15 season. Moreover, no significant difference was found for the H1N1-predominant 2013-14 season, in contrast to the results of observational studies during the 2013-14 season. For influenza B viruses, LAIV3 provided better protection when all three seasons were pooled (HR=0.66, 95%CI 0.46—0.96). This was driven primary by better protection with LAIV3 during the 2013-14 season (HR 0.14, 95%CI 0.03—0.72). No significant difference in protection against influenza B viruses was noted for the 2012-13 or 2014-15 seasons.
Summary
In pooled analyses from observational studies, LAIV demonstrated some statistically significant effectiveness against all influenza A and B compared with no vaccine. Effectiveness was also significant, but lower, against influenza A(H1N1)pdm09 viruses when U.S. and non-U.S. studies were pooled; but when analyzed separately effectiveness was statistically significant only for the non-U.S. studies. In terms of relative odds for influenza infection for those receiving LAIV compared with IIV, IIV was more effective against influenza A(H1N1)pdm09 viruses. For influenza B viruses, the odds ratio favored LAIV, but the difference was not statistically significant. LAIV and IIV performed similarly against influenza A(H3N2) viruses.
References
- Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008 Aug 8;57(RR-7):1-60.
- Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007 Feb 15;356(7):685-96.
- Ashkenazi S, Vertruyen A, Aristegui J, Esposito S, McKeith DD, Klemola T, et al. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J. 2006 Oct;25(10):870-9.
- Advisory Committee on Immunization Practices (ACIP). Summary report: June 22-23, 2016 (Meeting minutes). Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2016.
- Gaglani M, Pruszynski J, Murthy K, Clipper L, Robertson A, Reis M, et al. Influenza Vaccine Effectiveness Against 2009 Pandemic Influenza A(H1N1) Virus Differed by Vaccine Type During 2013-2014 in the United States. J Infect Dis. 2016 May 15;213(10):1546-56.
- Advisory Committee on Immunization Practices summary report: February 26, 2015 (Meeting minutes). Atlanta, GA: US Department of Health and Human Services, CDC; 2015.
- Grohskopf LA, Sokolow LZ, Broder KR, Olsen SJ, Karron RA, Jernigan DB, et al. Prevention and Control of Seasonal Influenza with Vaccines. MMWR Recomm Rep. 2016 Aug 26;65(5):1-54.
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Bresee JS, Fry AM, et al. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices – United States, 2017-18 Influenza Season. MMWR Recomm Rep. 2017 Aug 25;66(2):1-20.
- Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016 Oct 12;355:i4919.
- Kwong JC, Pereira JA, Quach S, Pellizzari R, Dusome E, Russell ML, et al. Randomized evaluation of live attenuated vs. inactivated influenza vaccines in schools (RELATIVES) cluster randomized trial: Pilot results from a household surveillance study to assess direct and indirect protection from influenza vaccination. Vaccine. 2015 Sep 11;33(38):4910-5.
- Loeb M, Russell ML, Manning V, Fonseca K, Earn DJ, Horsman G, et al. Live Attenuated Versus Inactivated Influenza Vaccine in Hutterite Children: A Cluster Randomized Blinded Trial. Ann Intern Med. 2016 Nov 1;165(9):617-24.
- Caspard H, Gaglani M, Clipper L, Belongia EA, McLean HQ, Griffin MR, et al. Effectiveness of live attenuated influenza vaccine and inactivated influenza vaccine in children 2-17 years of age in 2013-2014 in the United States. Vaccine. 2016 Jan 2;34(1):77-82.
- Chung JR, Flannery B, Thompson MG, Gaglani M, Jackson ML, Monto AS, et al. Seasonal Effectiveness of Live Attenuated and Inactivated Influenza Vaccine. Pediatrics. 2016 Feb;137(2):e20153279.
- Helmeke C, Grafe L, Irmscher HM, Gottschalk C, Karagiannis I, Oppermann H. Effectiveness of the 2012/13 trivalent live and inactivated influenza vaccines in children and adolescents in Saxony-Anhalt, Germany: a test-negative case-control study. PLoS One. 2015;10(4):e0122910.
- Jackson ML, Chung JR, Jackson LA, Phillips CH, Benoit J, Monto AS, et al. Influenza Vaccine Effectiveness in the United States during the 2015-2016 Season. N Engl J Med. 2017 Aug 10;377(6):534-43.
- McLean HQ, Thompson MG, Sundaram ME, Kieke BA, Gaglani M, Murthy K, et al. Influenza vaccine effectiveness in the United States during 2012-2013: variable protection by age and virus type. J Infect Dis. 2015 May 15;211(10):1529-40.
- McLean HQ, Caspard H, Griffin MR, Poehling KA, Gaglani M, Belongia EA, et al. Effectiveness of live attenuated influenza vaccine and inactivated influenza vaccine in children during the 2014-2015 season. Vaccine. 2017 May 9;35(20):2685-93.
- Ohmit SE, Thompson MG, Petrie JG, Thaker SN, Jackson ML, Belongia EA, et al. Influenza vaccine effectiveness in the 2011-2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis. 2014 Feb;58(3):319-27.
- Ohmit SE, Petrie JG, Malosh RE, Johnson E, Truscon R, Aaron B, et al. Substantial Influenza Vaccine Effectiveness in Households With Children During the 2013-2014 Influenza Season, When 2009 Pandemic Influenza A(H1N1) Virus Predominated. J Infect Dis. 2016 Apr 15;213(8):1229-36.
- Pebody R, Warburton F, Andrews N, Ellis J, von Wissmann B, Robertson C, et al. Effectiveness of seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2014/15 end of season results. Euro Surveill. 2015;20(36).
- Pebody R, Warburton F, Ellis J, Andrews N, Potts A, Cottrell S, et al. Effectiveness of seasonal influenza vaccine for adults and children in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2015/16 end-of-season results. Euro Surveill. 2016 Sep 22;21(38).
- Pebody R, Warburton F, Ellis J, Andrews N, Potts A, Cottrell S, et al. End-of-season influenza vaccine effectiveness in adults and children, United Kingdom, 2016/17. Euro Surveill. 2017 Nov;22(44).
- Poehling KA, Caspard H, Peters TR, Belongia EA, Congeni B, Gaglani M, et al. 2015-2016 Vaccine Effectiveness of Live Attenuated and Inactivated Influenza Vaccines in Children in the United States. Clin Infect Dis. 2018 Feb 10;66(5):665-72.
- Skowronski DM, Chambers C, Sabaiduc S, De Serres G, Winter AL, Dickinson JA, et al. Integrated Sentinel Surveillance Linking Genetic, Antigenic, and Epidemiologic Monitoring of Influenza Vaccine-Virus Relatedness and Effectiveness During the 2013-2014 Influenza Season. J Infect Dis. 2015 Sep 1;212(5):726-39.
- Skowronski DM, Chambers C, Sabaiduc S, De Serres G, Winter AL, Dickinson JA, et al. Beyond Antigenic Match: Possible Agent-Host and Immuno-epidemiological Influences on Influenza Vaccine Effectiveness During the 2015-2016 Season in Canada. J Infect Dis. 2017 Dec 19;216(12):1487-500.
- Zimmerman RK, Nowalk MP, Chung J, Jackson ML, Jackson LA, Petrie JG, et al. 2014-2015 Influenza Vaccine Effectiveness in the United States by Vaccine Type. Clin Infect Dis. 2016 Dec 15;63(12):1564-73.
Literature Search Strategy
- Medline (OVID) 1946-
- (Influenza OR flu) AND (((live OR attenuated OR intranasal OR nasal OR cold adapted) ADJ5 (vaccin* OR immun*)) OR CAIV OR LAIV OR FluMist OR Fluenz)
- NOT exp animals/ not exp humans/
- Limit English only; 2011-current (Nov 2016-current); All children
- Embase (OVID) 1947-
- (Influenza OR flu) AND (((live OR attenuated OR intranasal OR nasal OR cold adapted) ADJ5 (vaccin* OR immun*)) OR CAIV OR LAIV OR FluMist OR Fluenz)
- NOT exp animals/ not exp humans/
- Limit English only; 2011-current (Nov 2016-curent);
- limit to (child <unspecified age> or preschool child <1 to 6 years> or school child <7 to 12 years> or adolescent <13 to 17 years>)
- CINAHL (Ebsco) 1982-
- (Influenza OR flu) AND (((live OR attenuated OR intranasal OR nasal OR “cold adapted”) N5 (vaccin* OR immun*)) OR CAIV OR LAIV OR FluMist OR Fluenz)
- Limiters – Published Date: 20110101-20161231 (20161101-20171031); English Language; Age Groups: Child, Preschool: 2-5 years, Child: 6-12 years, Adolescent: 13-18 years
- Cochrane Library
- (Influenza OR flu) AND (((live OR attenuated OR intranasal OR nasal OR “cold adapted”) NEAR/5 vaccin*) OR ((live OR attenuated OR intranasal OR nasal OR “cold adapted”) NEAR/5 vaccin*) OR CAIV OR LAIV OR FluMist OR Fluenz)
- 2011-2016 (Nov 1 2016-Oct 31 2017)
- Clinical Trials.gov
- (influenza vaccine AND (attenuated OR live OR nasal spray OR intranasal OR cold-adapted)) OR Flumist OR Fluenz| Child | Studies updated from 01/01/2011 to 09/30/2016 (Studies updated from 11/01/2016 to 10/31/2017)
- Scopus
- TITLE-ABS-KEY((Influenza OR flu) AND ((live W/3 vaccin*) OR (attenuated W/3 vaccin*) OR (intranasal W/3 vaccin*) OR (nasal W/3 vaccin*) OR (“cold adapted” W/3 vaccin*) OR (live W/3 immun*) OR (attenuated W/3 immun*) OR (intranasal W/3 immun*) OR (nasal W/3 immun*) OR (“cold adapted” W/3 immun*) OR CAIV OR LAIV OR FluMist OR Fluenz)) AND TITLE-ABS-KEY(child* OR adolescent* OR toddler*) AND NOT INDEX(medline)
* The first search was performed on 1 December 2016, and included work published from 2011 forward. The second search was performed on 31 October 2017, and included work published between November 2016 through October 2017. Date windows for the second search are in parentheses.
Abbreviations: ICICLE=Influenza Clinical Investigation for Children; LAIV3=trivalent live attenuated influenza vaccine; LAIV4=quadrivalent live attenuated influenza vaccine; TNCC=Test negative case-control; US Flu VE=U.S. Influenza Vaccine Effectiveness Network.
*Instances where risk of bias was judged to be “Serious” all lacked adjustment for a potential confounder of interest (ROBINS-I Domain 1). Potential confounders of interest included age, geographic site (for multisite studies), season (for multiseason studies), presence of asthma (adjustment for high-risk medical condition accepted as proxy for asthma if asthma included among those conditions), and calendar time of illness onset.
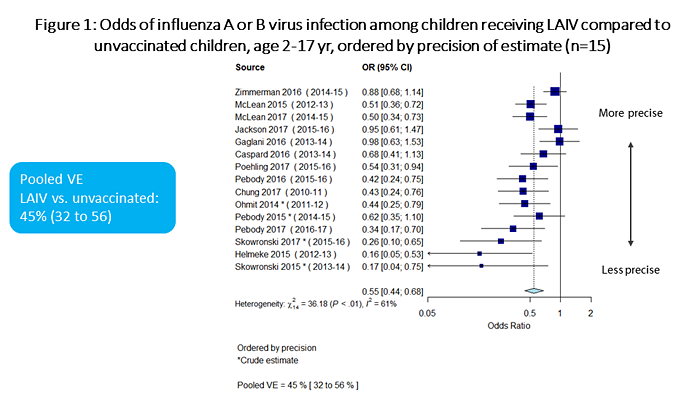
Figure 1 is a plot which summarizes the odds of influenza A or B virus infection among children receiving LAIV vs. unvaccinated children, ordered by precision of estimate. There are a total of 15 individual estimates. The pooled odds ratio is 0.55 (95%CI 0.44-0.68).
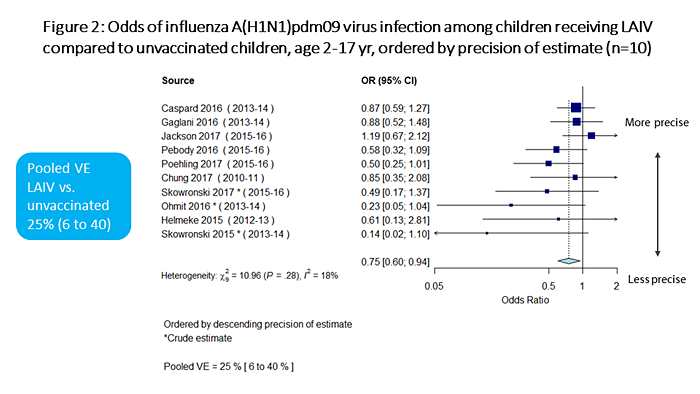
Figure 2 is a plot summarizing the odds of influenza A(H1N1)pdm09 virus infection among children receiving LAIV vs. unvaccinated children, ordered by precision of estimate. There are a total of 10 individual estimates. The pooled odds ratio is 0.75 (95%CI 0.60-0.94).
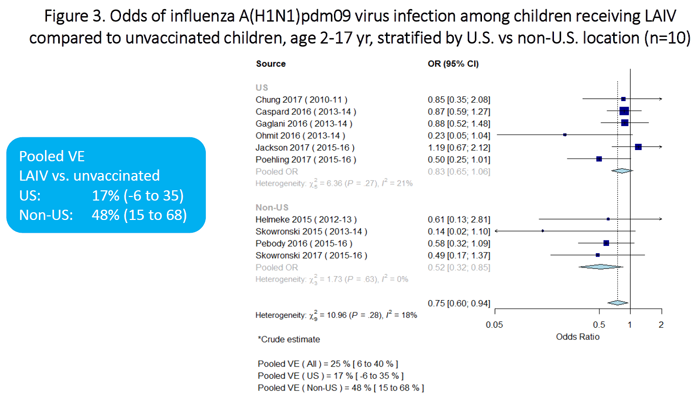
Figure 3 is a plot which summarizes the odds of influenza A(H1N1)pdm09 virus infection among children receiving LAIV vs. unvaccinated children, stratified by location (U.S. vs non-U.S.). There are a total of 10 individual estimates (6 for U.S. studies and 4 for non-U.S. studies). The pooled odds ratio for the U.S. studies is 0.83 (95%CI 0.65-1.06). The pooled odds ratio for the non-U.S. studies is 0.52 (95%CI 0.32-0.85).
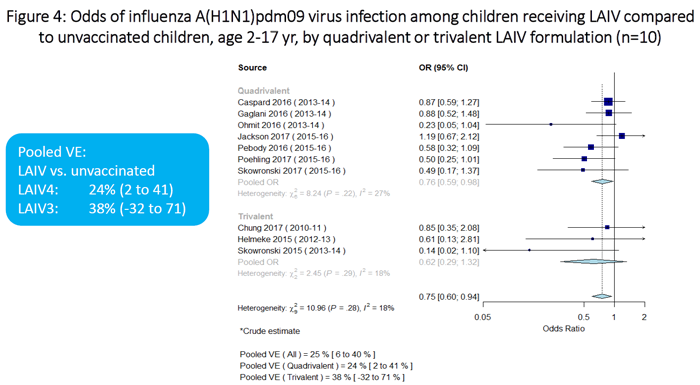
Figure 4 is a plot on which summarizes the odds of influenza A(H1N1)pdm09 virus infection among children receiving LAIV vs. unvaccinated children, stratified by LAIV formulation (quadrivalent vs trivalent). There are a total of 10 individual estimates (7 for quadrivalent and 3 for trivalent). The pooled odds ratio for the quadrivalent estimates is 0.76 (95%CI 0.59-0.98). The pooled odds ratio for the trivalent estimates is 0.62 (95%CI 0.29-1.32).
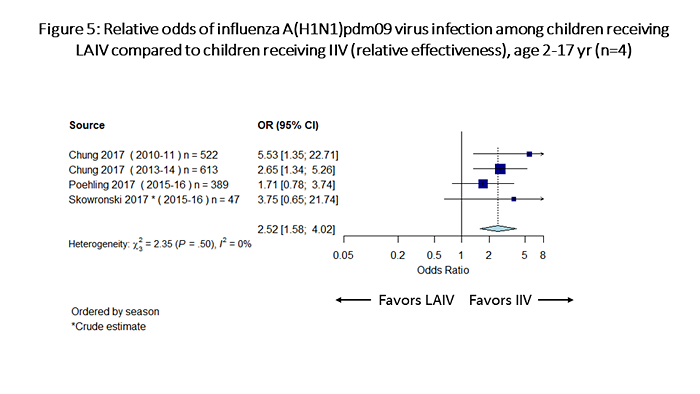
Figure 5 is a plot which summarizes odds of influenza A(H1N1)pdm09 infection among children receiving LAIV vs children receiving IIV (relative effectiveness). There are a total of 4 individual estimates. The pooled odds ratio is 2.52 (95%CI 1.58-4.02).
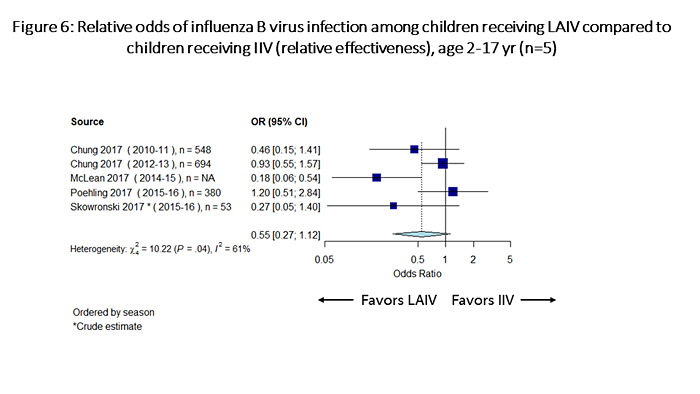
Figure 6 is a plot which summarizes odds of influenza B infection among children receiving LAIV vs children receiving IIV (relative effectiveness). There are a total of 5 individual estimates. The pooled odds ratio is 0.55 (95%CI 0.27-1.12).
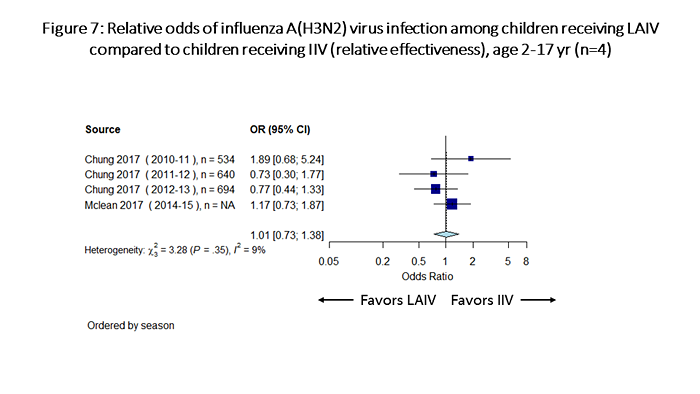
Figure 7 is a plot which summarizes odds of influenza A(H3N2) infection among children receiving LAIV vs children receiving IIV (relative effectiveness). There are a total of 4 individual estimates. The pooled odds ratio is 1.01 (95%CI 0.73-1.38).
