Recommendations for Fully Vaccinated People
COVID-19 Homepage
Global COVID-19 Budget Factsheet
Fact Sheet: CDC’s global response to COVID-19 Pdf[584 KB, 4 pages] (For Print)
CDC’s ongoing global response to the COVID-19 pandemic reflects and builds on decades of global health partnerships. While the world is benefiting from the rapid development and deployment of effective vaccines, the emergence of variants has further emphasized the importance of vaccination, boosters, and ongoing prevention efforts to protect against COVID-19. To contribute to the global goal to vaccinate 70% of the world against COVID-19 by the end of 2022, the United States launched the Initiative for Global Vaccine Access (Global VAX), a whole-of-government effort to ensure that eligible populations receive COVID-19 vaccines. CDC is a key contributor to Global VAX Pdf[565KB, 2 pages] and is also working to address inadequate surveillance and laboratory capacity to prevent overburdening of health systems.
CDC’s Response Goals and Objectives
CDC’s global response builds on its Strategy for Global Response to COVID-19 (2020-2023) to define program priorities, develop monitoring and evaluation criteria, and set targets for impact. The U.S. COVID-19 Global Response and Recovery Framework Pdf[317KB, 12 pages]External provides overarching goals and objectives to end the pandemic, mitigate wider harms to people and societies, and strengthen global recovery and readiness for future pandemic threats.
CDC’s Strategy for Global Response to COVID-19 (2020–2023) are to:
-
- Strengthen capacity to plan for and deliver COVID-19 vaccines, and to evaluate vaccines and vaccination programs using timely, accurate data (Global VAX);
- Strengthen capacity at country and regional levels to prevent, detect, and respond to COVID-19 cases and future pandemic threats by:
- Strengthening the global public health workforce
- Strengthening surveillance and laboratory systems and modernizing data systems
- Prevent and mitigate COVID-19 transmission across borders, in communities, in healthcare facilities, and among healthcare workers;
- Contribute to the scientific understanding of COVID-19 and other pandemic and emerging threats and address critical unknowns;
- Strengthen the global health architecture working with multilateral and multisectoral partners to augment surveillance, laboratories, alert systems and capacities for early and effective prevention, detection, and response to potential health emergencies.
Download: CDC Strategy for Global Response to COVID-19 2020 – 2023 Pdf[302KB, 6 pages] (For Print)
CDC in Action: COVID-19 Response Activities
CDC’s on-the-ground presence in more than 60 countries has been critical for supporting ministries of health as they respond to COVID-19. Working side-by-side with country partners, CDC provides response coordination, surveillance, diagnostic capacity, genomic sequencing, infection prevention and control, and vaccine administration support among other activities.
Support response coordination in more than 30 countries
In Sierra Leone, CDC supported adaptation of a system for electronic data capture in the field, allowing for more rapid access to higher quality surveillance data.
Support epidemiologic response in more than 30 countries, including seroprevalence and other studies, technical assistance, and acute febrile and mortality surveillance.
In Zambia, CDC supported early case investigation and contact tracing, which were critical initial response activities.
Support laboratory testing in more than 50 countries
In Malawi, CDC provided technical support for setting up a COVID-19 testing laboratory, helped identify CDC-supported HIV laboratories for additional COVID-19 testing, and developed a 3-day training course on biosafety standards.
Investigate variants
CDC is providing supplies and training to augment sequencing capacity in more than 30 countries, which will increase availability of information about new variants and their impact.
CDC supports vaccine administration in more than 70 countries worldwide
Vaccine distribution
In Nigeria, CDC helped the government develop its plan for distributing the vaccines as they became available.
Vaccine workforce development
In Belize, CDC supported the Ministry of Health and Wellness to increase the number of trained staff who administer COVID-19 vaccines. The surge workforce has been instrumental in Belize, inoculating 50.4 percent of the eligible population against COVID-19 in 2021.
In Mali, CDC and its partners supported the government in training more than 800 healthcare workers involved in COVID-19 vaccination activities.
Enhance pan-respiratory surveillance in more than 30 countries
In Thailand, CDC supported genetic sequencing efforts of SARS-CoV-2 by providing technical assistance and integrating COVID-19 surveillance into the influenza sentinel surveillance system, helping Thailand evaluate vaccine effectiveness for COVID-19 vaccines.
Support training for IPC in more than 27 countries
In Vietnam, CDC supported training on IPC, including testing for laboratory and hospital staff at national and provincial hospitals in more than 30 provinces.
Global Response Program Overview
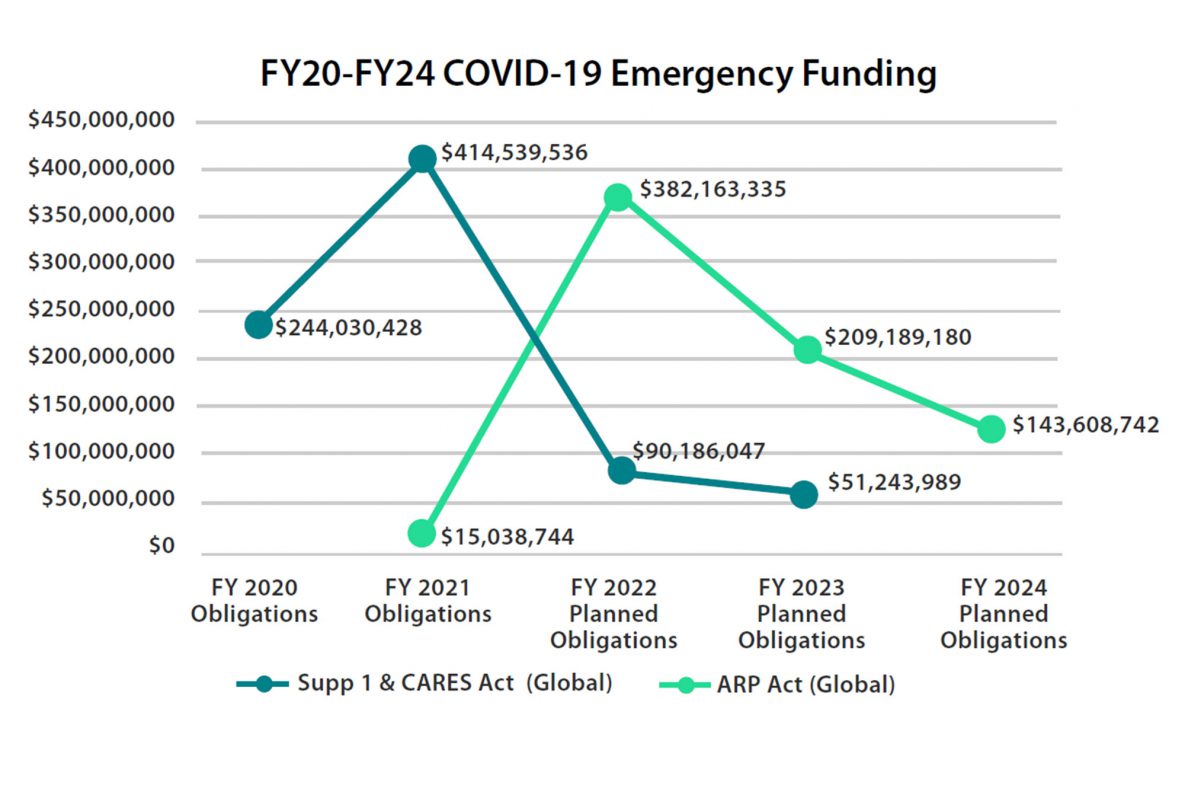
CDC’s global response to the COVID-19 pandemic is supported in part by the Coronavirus Preparedness and Response Supplemental Appropriations Act 2020 (Public Law 116-123External), the Coronavirus Aid, Relief, and Economic Security (CARES) Act 2020 (Public Law 116-136External), and the American Rescue Plan (ARP) Act 2021 (Public Law 117-2External).
From the beginning of its response to the COVID-19 pandemic, CDC leveraged decades-long partnerships with ministries of health and existing CDC global programmatic assets on the ground to immediately mobilize technical assistance and support at the country level. These include previous investments in global health security and programs such as the President’s Emergency Fund for AIDS Relief (PEPFAR), the President’s Malaria Initiative (PMI), polio eradication, preparations for influenza and other pandemic diseases, and outbreak response.
CDC is leveraging preparedness, response, and capacity investments to ensure quick access to response funds at the community level, while assessing the needs of countries, regions, and U.S.-based efforts to respond effectively as the pandemic evolves. CDC monitors the COVID-19 pandemic and directs resources based on changes to the disease epidemiology, response requirements, and opportunities to better understand the evolving virus.
CDC Global COVID-19 Funding by Strategic Priorities*
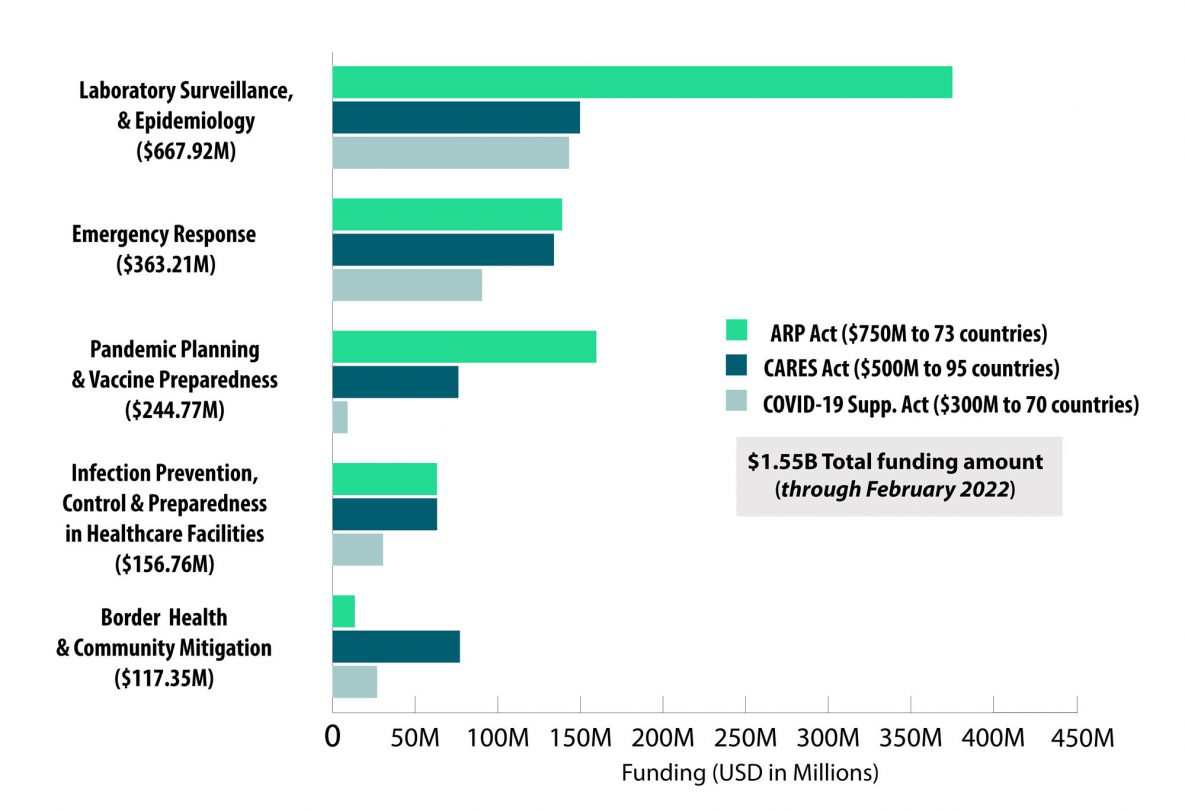
*includes $140M for Strengthening Data Systems
CDC Global COVID-19 Funding at a Glance
With the provision of $800 million in global funding from the Coronavirus Preparedness and Response Supplemental Appropriations and CARES Acts, CDC:
- Organized and executed support for rapid response teams, laboratory and reagents, surveillance systems, and infection prevention and control enhancements at facilities treating COVID-19 cases,
- Expanded border screening assistance,
- Supported evaluations to characterize SARS-CoV-2 (the virus that causes COVID-19), and
- Responded to a broad range of technical assistance requests.
With the provision of $750 million in global funding from the 2021 ARP Act, CDC is working to advance core initiatives including:
- Global VAX,
- Enhanced global respiratory surveillance, and Continued enhancements to global public health data.