WHO Region of the Americas (AMR) 2011-2012
As of FY 2011, there are four bilateral influenza cooperative agreements in the Region of the Americas. These agreements with ministries of health (MOH) or institutions designated by the MOHs work with the Pan American Health Organization (PAHO)/the World Health Organization (WHO) and the U.S. Centers for Disease Control and Prevention (CDC) to build capacity to routinely identify and respond to seasonal and novel influenza strains across the Americas.
CDC direct country support via cooperative agreements is established in the following countries:
In addition, CDC supports PAHO via a cooperative agreement. CDC also supports activities with the Center for Central America and Panama (CDC-CAP) [892 KB, 4 pages] at the CDC, Global Disease Detection (GDD) site in Guatemala. These activities support programs in eight Central American/Caribbean countries including Belize, Guatemala, El Salvador, Honduras, Nicaragua, Costa Rica, Panama, and the Dominican Republic.
The core activities of our bilateral agreements and technical assistance are:
- To build sustainable national capacity to identify and respond to seasonal influenza, pandemic influenza and other emerging diseases in accordance with International Health Regulations 2005 (IHR).
- To make routine contributions of surveillance and virology data to WHO’s Global Influenza Surveillance and Response System (GISRS).
- To increase the geographic reach of WHO GISRS.
- To provide earlier access to critical virus isolates from humans and birds for WHO GISRS.
- To increase the numbers of shipments and influenza isolates provided by local influenza labs for analysis by WHO Collaborating Centers (CC).
- To develop sustainable epidemiologic and virologic surveillance systems for severe influenza in order to gain understanding of the disease and economic burden caused by influenza and other respiratory viruses.
- To develop and sustain interagency national preparedness plans.
- To develop and train local rapid response and containment teams.
- To sustain and leverage quality sentinel surveillance and study cohorts to explore the potential cost-effectiveness of expanding vaccination and incorporating new delivery mechanisms, formulations, and novel influenza vaccines in the PAHO Region.
In addition to our bilateral work, we also partner with the U.S. Naval Medical Research Unit No. 6 (NAMRU-6) in Lima, Peru to jointly support South American countries that are starting influenza surveillance.
Sara Mirza, PhD, MPH (from December 2011)
Epidemiologist
Extramural Program
Influenza Division, NCIRD
U.S. Centers for Disease Control and Prevention
Email: smirza@cdc.gov
Eduardo Azziz-Baumgartner, MD, MPH
Medical Officer/Epidemiologist
International Epidemiology and Research Team
Influenza Division, NCIRD
U.S. Centers for Disease Control and Prevention
Email: eha9@cdc.gov
Tomas Rodriguez, MA (until May 2012)
Public Health Advisor
Extramural Program
Influenza Division, NCIRD
U.S. Centers for Disease Control and Prevention
Email: trr0@cdc.gov
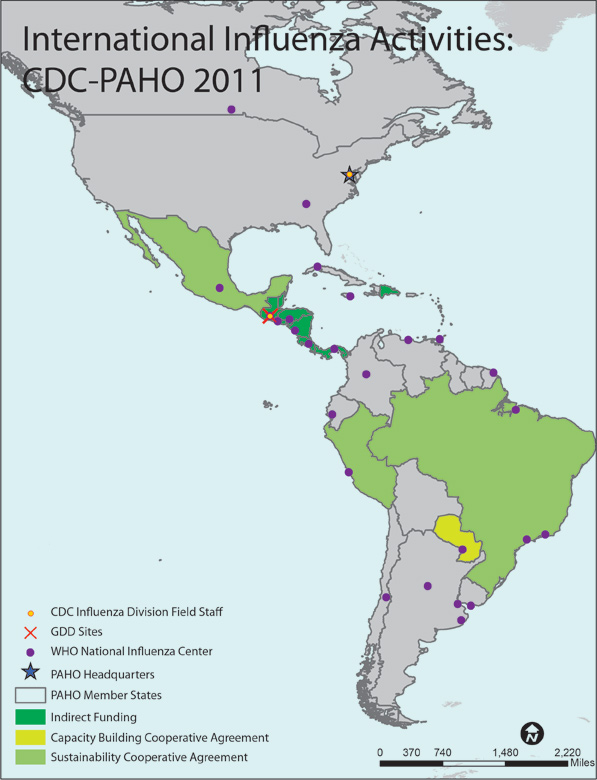
A map of the WHO Region of the Americas (AMR) shows all 46 AMR member states/countries. The member countries, outlined with gray borders, include Antigua, Argentina, Aruba, Barbados, Barbuda, Belize, Bermuda, Bolivia, Bonaire, Brazil, British Virgin Islands, Canada, Cayman Islands, Chile, Columbia, Costa Rica, Cuba, Curacao, Dominica, Dominican Republic, Ecuador, El Salvador, French Guyana, Grenada, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Jamaica, Martinique, Mexico, Montserrat, Netherland Antilles, Nicaragua, Panama, Paraguay, Peru, Puerto Rico, St. Lucia, St. Vincent and the Grenadines, Suriname, Trinidad and Tobago, Turks and Caicos, United States of America, Uruguay, and Venezuela.
Countries with shading indicate that the Influenza Division provides project funding and technical assistance through cooperative agreements. Paraguay is shaded yellow to indicate that they have a Capacity Building Cooperative Agreement. Brazil, Mexico and Peru are shaded green to indicate that they have Sustainability Cooperative Agreements. Belize, Costa Rica, Dominican Republic, El Salvador, Guatemala, Honduras, Nicaragua and Panama are shaded dark green to indicate that they receive indirect funding from the Division.
CDC Influenza Division Field Staff, indicated by a yellow dot outlined in red, are located in the following cities: Guatemala City and Washington, DC.
The Global Disease Detection [GDD] Site, indicated by the red “X”, is located in Guatemala.
WHO National Influenza Centers (NICs), indicated by a purple dot, are located in Argentina (Buenos Aires, Córdoba, and Mar Del Plata), Brazil (Ananindeua, Sao Paulo, and Rio de Janeiro), Canada (Winnipeg), Chile (Santiago), Columbia (Bogota), Costa Rica (Cartago), Cuba (Havana), Ecuador (Guayaquil), El Salvador (San Salvador), France-French Guiana (Cayenne), Guatemala (Barcenus Villa Nueva), Honduras (Tegucigalpa), Jamaica (Kingston), Mexico (Mexico City), Nicaragua (Managua), Panama (Panama City), Paraguay (Asunción), Peru (Lima), Trinidad and Tobago (Port of Spain), Uruguay (Montevideo), the United States of America (Atlanta, Georgia), and Venezuela (Caracas).
The Pan American Health Organization (PAHO) Headquarters, indicated by a blue star, is located in Washington, DC (USA).
- The PAHO Nationwide Enhanced SARI Surveillance Protocol was developed.
- PAHO is working with an information technology specialist who has developed a web-based data entry and analysis system that generates automated outputs of integrated laboratory and clinical data. This system is being scaled-up for replication initially in at least two other Member States.
- PAHO missions to Bolivia, Dominican Republic, Ecuador, Guatemala, and Nicaragua were carried out to provide technical assistance on appropriate practices for infection prevention and control of pandemic influenza in health care centers and on clinical management of severe and critically ill cases. During the missions, PAHO provided training on infection prevention and control practices, including the correct use of the personal protective equipment (PPE) for health care workers.
- Questionnaires were developed to assess the risks to health care personnel and piloted in Mexico.
- Guidelines on epidemiological surveillance of health care associated infections, specifically ventilator-associated pneumonia were developed and reviewed by regional experts. Implementation of the guidelines will follow training courses in El Salvador, Paraguay, Trinidad and Tobago, and Uruguay. Chile, Colombia, and the Dominican Republic are interested in utilizing the guidelines as well.
- Central to all the post-pandemic evaluations is the need to include and support risk communication as a public health tool. Countries are evaluating the results of studies assessing perceptions to create messages to reach targeted audiences. Countries also expanded training to address all public health emergencies.
- PAHO tested a pilot risk communication International Health Regulations 2005 (IHR) simulation during a three-day workshop and published all PAHO training materials in risk communication on an online site at www.paho.org/riskcomm.
U.S. CDC Direct WHO Regional Office Support
Technical cooperation activities initially centered on influenza and pandemic influenza preparedness through the strengthening of three pillars; preparedness and communication, surveillance and detection, and response and containment. Post influenza pandemic, several key issues were identified which translated into important lessons learned.
First, the varied capacity of countries to detect unusual health events was noted. Second, the collapse during the pandemic of established sentinel surveillance systems to monitor influenza-like illness (ILI) and severe acute respiratory infection (SARI) due in large part to the overwhelming demand for clinical services and a lack of integration of these sentinel surveillance activities within the health care services sector. Third, public health laboratories, which had been trained and equipped to diagnose influenza with multiple diagnostic techniques, were also overwhelmed, due in large part to the fact that they were not being used for surveillance purposes, but rather for clinical diagnostic purposes. These lessons learned refocused PAHO’s technical cooperation with the U.S. Centers for Disease Control and Prevention’s (CDC) support to strengthen national SARI surveillance, integration between epidemiologic and virologic data, and obtain a better understanding of the mortality due to influenza during the pandemic.
During the 2009 influenza pandemic the surveillance of severe respiratory disease cases became increasingly important. This was because these cases in a hospital setting, are easier to capture, are smaller in number than the milder ambulatory cases, and during a pandemic, information about severe cases is of paramount importance for making decisions about response. The first step taken was to draft guidelines based upon the PAHO-CDC Generic Protocol for Influenza Surveillance (GPIS), but focused on strengthening SARI surveillance. Next, the tasks of sensitizing the countries and implementing the protocol proved to be relatively easy, as countries had identified this lack of data on severe cases to be important and were eager to improve their SARI surveillance. In 2010, this protocol was implemented in selected Caribbean countries, through technical cooperation with the Caribbean Epidemiology Centre (CAREC) and also in the Southern Cone in Uruguay and implementation was ongoing in Chile, Honduras, and Paraguay in 2011. Additionally, in 2011, Colombia, Ecuador, and Peru developed work plans to establish this surveillance and several other countries are considering adopting the strategy as well.
Surveillance Activities
- A specific protocol, the PAHO Nationwide Enhanced SARI Surveillance Protocol, which further develops the concepts and importance of SARI surveillance, was developed.
- SARI surveillance was established in six hospitals in Paraguay, which are providing weekly clinical and laboratory data to PAHO.
- SARI surveillance was established in the national hospital in Barbados, Dominica, Jamaica, St. Lucia, St. Vincent and the Grenadines, Trinidad and Tobago and weekly laboratory and clinical data are being provided to the CAREC/PAHO.
- SARI surveillance was established in all hospitals in Uruguay with an intensive care unit and 80% of the remaining hospitals and real-time linked clinical and virologic information is available online. Visit Uruguay's Ministry of Public Health website for additional information.
Considering the challenges faced during the pandemic, technical cooperation was directed to improve the capacity in the laboratory to process specimens for real-time RT-PCR, through the purchase of automated extractors and vacuum extractors. PAHO has continued to support the strengthening of laboratory capacity for the diagnosis of influenza and other respiratory viruses, including through the limited decentralization of real-time RT-PCR for influenza, through refresher courses for real-time RT-PCR and immunofluorescence, and through participation in the WHO External Quality Assessment Project (EQAP). Through these activities, PAHO continues to strengthen the regional laboratory network, which now consists of 23 National Influenza Centers (NIC) in Latin America and the Caribbean.
Laboratory Activities
- PAHO has been working with the regional laboratories to strengthen the diagnostic capabilities for influenza and other respiratory viruses, through the provision of supplies, equipment purchases, and training. Based on lessons learned during the 2009 pandemic, post-pandemic efforts have focused on the decentralization of real-time RT-PCR and immunofluorescence as well as automation of the extraction process for real-time RT-PCR.
PAHO has updated their emergency operation center (EOC) in Washington D.C. to coordinate activities and deploy rapid response teams (RRT) in the Region. As the point of contact, PAHO serves as a key communication link between the Ministries of Health (MOH) and technical assistance. PAHO continues to help all countries in creating situation rooms and EOCs to centralize data and coordinate preparedness activities.
Preparedness Activities
- PAHO is working with Peru to carry out a national and subnational evaluation of their core capacities for surveillance and response to public health emergencies under the IHR (2005) framework. Pandemic influenza will be used as the context for the evaluation and action plans for addressing the identified gaps will be a product of this meeting. This evaluation will continue to take place in other countries in South America.
- PAHO developed a two and a half day training course, in conjunction with the University of North Carolina at Chapel Hill to train laboratorians about influenza data analysis and dissemination. More than 45 persons were trained, representing all four subregions. The course is currently available online in both English and Spanish at http://cursos.campusvirtualsp.org/course/view.php?id=62.
- PAHO in conjunction with the Universidade de Norte in Colombia designed a one-day course to create tools to capture and sustain the work that has been done at the country level in risk communication to create a certificate program on the Virtual Campus for participants throughout Latin America. We also developed a field guide to capture the steps and lessons learned. Both products await English translation.
Otavio Oliva, MD, MPH
Advisor, Viral Diseases
Pan American Health Organization
Regional Office for the Americas
World Health Organization
Washington, DC
Email: olivaot@paho.org
Rakhee Palekar, MD, MPH
Medical Officer
U.S. Centers for Disease Control and Prevention
Washington, DC
Email: palekar@paho.org
Mauricio Cerpa, MD
Influenza Surveillance Specialist
Pan American Health Organization
Regional Office for the Americas
World Health Organization
Washington, DC
Email: cerpamau@paho.org
Oona Bilbao
Project Specialist
Pan American Health Organization
Regional Office for the Americas
World Health Organization
Washington, DC
Email: bilbaoon@paho.org
