Recent Work
Our Work - 2023
Responding domestically to China’s COVID-19 surge

CDC staff worked in late December to develop and launch a new travel requirement in response to the COVID-19 surge in China. Air passengers 2 years of age and older flying from China, Hong Kong, or Macau to the United States are required to get a test no more than two days before travel and show a negative test result to the airline upon their departure. CDC also posted a Level 2 Travel Health Notice for China, which means US travelers to China, Hong Kong, and Macau need to practice enhanced precautions to protect themselves and others from COVID-19.
In addition, CDC expanded the Traveler-based Genomic Surveillance program for early detection of new SARS-CoV-2 Variants by two new airports (Los Angeles and Seattle). This voluntary program serves as an early warning system to detect and characterize new and rare variants of the virus that causes COVID-19.
Uganda’s Ebola outbreak declared over
On January 11, the government of Uganda announced the end of the Ebola outbreak that began there in September 2022, resulting in 164 reported cases and 77 deaths in nine of the country’s districts. A key part of the domestic preparedness effort was enhancing capacity at Laboratory Response Network laboratories and establishing an entry screening and monitoring program for travelers arriving to the United States from Uganda; the screening program ended shortly after the outbreak was declared over. CDC will continue to work with Uganda on surveillance; infection prevention and control; laboratory diagnostics; survivor support including care and treatment; viral testing and research on viral persistence; and other activities to support rapid detection and response to any future cases and outbreaks of Ebola.
CDC-verified lab tests support US preparedness for Ebola
Laboratory readiness is an important piece of protecting the United States from the introduction and spread of Ebola and other emerging diseases. In 2019, CDC’s Laboratory Preparedness and Response Branch (LPRB) collaborated with the Department of Defense to deploy a new diagnostic test called the Next Generation Diagnostic System Warrior Panel (Warrior Panel) to the Laboratory Response Network (LRN). This diagnostic test detects the bacteria that cause anthrax, plague, tularemia, and Q fever and the viruses that cause Ebola and Marburg. When Sudan ebolavirus (SUDV) emerged in Uganda in 2022, LPRB increased US testing capabilities by providing instruments, tests, and verification materials to more LRN laboratories and 10 Regional Emerging Special Pathogen Treatment Centers (RESPTCs), located in each Health and Human Services (HHS) region. At this time, 30 LRN laboratories and six RESPTCs can use the Warrior Panel to test for Sudan ebolavirus. The team is collaborating with CDC, US Army Medical Research Institute of Infectious Disease, and FDA to validate a real-time diagnostic test for emergency use authorization to detect SUDV. This test will allow LRN laboratories in the United States to rapidly test many samples for SUDV.
Cholera outbreak continues in Haiti
Since the laboratory confirmation of two cases of cholera infection in the Port-au-Prince area on October 2, 2022, more than 28,000 suspected cholera cases have been reported throughout Haiti, 85% of patients have been hospitalized, and more than 450 people have died from the disease. CDC is working with partners to support Haiti’s Ministry of Public Health and Population in efforts to control the outbreak. Ongoing social unrest in the country has hampered outbreak control activities and reduced the supply of safe drinking water, resulting in many residents having to rely on unsafe sources and untreated water and increasing the risk for cholera transmission.
A recent MMWR article by CDC Epidemic Intelligence Service (EIS) officer Denisse Vega Ocasio, colleagues from CDC, and Haitian public health agencies describes the current outbreak of cholera in Haiti. The authors note that although cholera cases have declined, a multifaceted approach is urgently needed that uses stronger surveillance, timely case management, targeted oral cholera vaccination campaigns, risk communication and community engagement, and improved access to safe water and sanitation services and emergency water treatment.
Foodborne and animal contact disease outbreaks

CDC, with federal and state partners, is investigating several multistate foodborne and animal contact outbreaks, including the following:
- Listeria infections with unknown food source. As of February 15, there have been 11 illnesses and 10 hospitalizations from 10 states.
- Listeria infections linked to deli meat and cheese. As of November 9, there have been 16 illnesses, 13 hospitalizations, and one death from six states.
- Listeria infections linked to enoki mushrooms. As of January 27, there have been three illnesses and three hospitalizations from three states.
- Salmonella infections linked to alfalfa sprouts. As of December 30, there have been 15 illnesses and two hospitalizations from three states.
Celebrating winter holidays safely

Over the holiday season, CDC developed and disseminated food safety materials that showed proper thermometer placement in commonly consumed holiday meats, the dangers of leaving food out too long, and the importance of preventing foodborne illness. Topics included raw cookie dough, cooking for large groups, perishable food, cooking food thoroughly, raw eggs, safe internal temperatures, and food poisoning symptoms.
When food safety messages were tested with audiences, participants suggested CDC include a variety of meals consumed by Americans during the holidays. CDC communicators used this feedback to create materials that represented diverse cultures and foods. The feedback also indicated that people were less likely to refrigerate leftovers quickly and check the internal temperature of cooked food around the holidays when entertaining guests and making new dishes. For holiday messaging, CDC communicators focused on these key prevention behaviors. In messaging about the food safety during the Super Bowl, CDC continued to highlight these important prevention steps and represent a diverse array of individuals.
CDC surveillance data help shape FDA policy
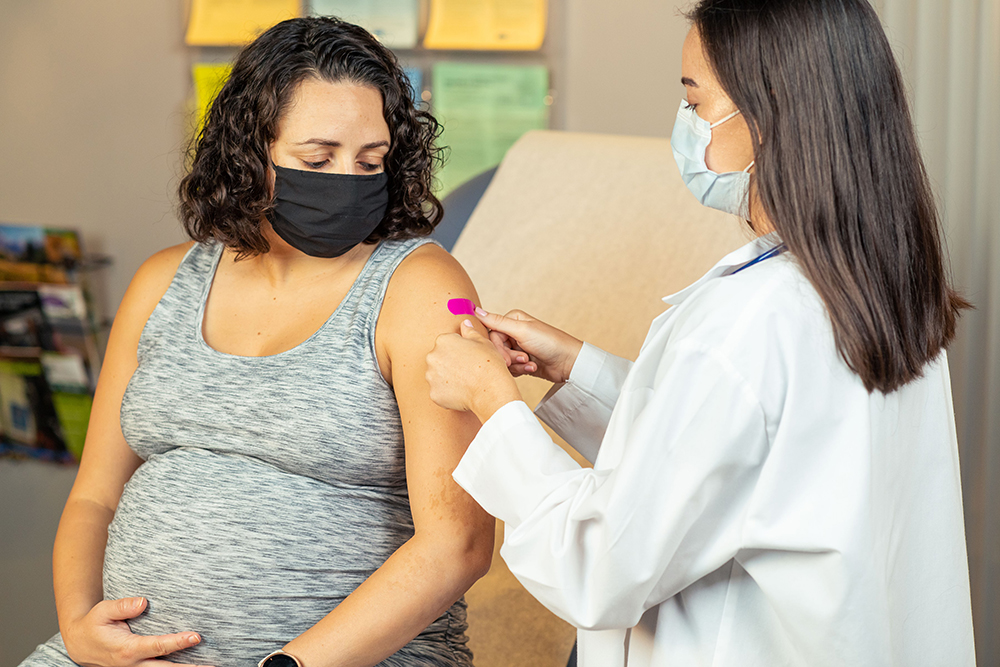
Since 2012, CDC has recommended Tdap vaccination in the third trimester of each pregnancy to prevent pertussis in infants younger than two months of age. Although Tdap vaccines have been licensed in the United States for use in persons aged 7 years and older, Tdap vaccination during pregnancy was an off-label recommendation. FDA recently approved both Tdap vaccines, Boostrix (GlaxoSmithKline) and Adacel (Sanofi Pasteur), for use during pregnancy to prevent pertussis in infants based on a re-analysis of the relevant data from an observational case-control study of the effectiveness of Tdap vaccine. CDC’s Emerging Infections Program staff conducted the original analysis that led to this historic approval. This is the first time that FDA has used real-world, observational data to approve a vaccine.
CDC adds to evidence linking alpha-gal syndrome to tick bites
CDC’s important work on alpha-gal syndrome recently received some attention. Rickettsial Zoonoses Branch (RZB) Chief Gilbert Kersh was interviewed for a story in Healio about a paper he co-authored with others at CDC, “Tick bite as a risk factor for alpha-gal–specific immunoglobulin E antibodies and development of alpha-gal syndrome,” published in the November 26 issue of Annals of Allergy, Asthma & Immunology. The story notes how RZB set up a case-control study to see if the suspected relationship between tick bites and alpha-gal syndrome would hold up, since previously the relationship was largely based on case reports and anecdotal evidence. The study found a strong association between self-reported tick bites, behaviors related to tick bites, and a diagnosis of alpha-gal syndrome. RZB has more work planned to better understand the distribution and burden of this emerging tickborne allergy.
Laws that increase availability of raw milk are linked to more illnesses, CDC study shows

An article co-written by CDC staff, “Foodborne illness outbreaks linked to unpasteurized milk and relationship to changes in state laws—United States, 1998–2018,” reports the number of outbreaks and outbreak-associated illnesses linked to unpasteurized (raw) milk over time. The article also compares those numbers in states where the sale of raw milk is legal with the numbers in states where it is prohibited.
The study found that the number of outbreaks linked to raw milk has increased over time. From 1998 through 2018, 202 outbreaks and 2,645 outbreak-associated illnesses occurred because of drinking raw milk. Areas where raw milk was legally sold had 3.2 times more outbreaks than areas where the sale of raw milk was illegal. Areas where raw milk was allowed to be sold in retail stores had 3.6 times more outbreaks than areas where sale was allowed only on farms. The study shows that laws that increase the availability of raw milk are associated with more illnesses and outbreaks. For more information about pasteurization and the health impacts of raw milk, visit our raw milk website.