4.3 Congenital anomalies of the nervous system: Microcephaly
‹View Table of Contents
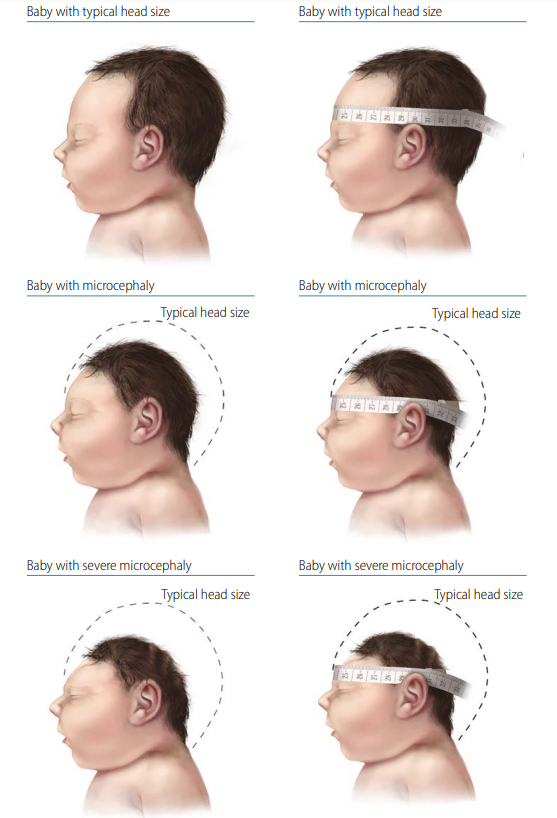
Microcephaly (microcephalus) describes a cranial vault that is smaller than normal for the infant’s sex and gestational age at birth (congenital). The size of the cranial vault is an indicator of the size of the underlying brain. Microcephaly can be diagnosed prenatally but is more often diagnosed after delivery. Fig. 4.12 shows newborns with normal head size, microcephaly, and severe microcephaly, and the correct position of a tape to measure the head circumference.
Fig. 4.12. Microcephaly
Relevant ICD-10 codes
Q02 Microcephaly
Diagnosis
Prenatal. Transabdominal ultrasound between 18 and 37 weeks’gestation might identify a small head size, with serial ultrasounds showing poor growth over time. Measurement of the fetal head circumference (HC) and biparietal diameter should be made on the axial image through the thalami at the level of the cavum septi pellucidi.
Postnatal. At delivery, a measurement of the occipito-frontal circumference or HC that is three standard deviations below the mean for the age- and sex-appropriate distribution curves is diagnostic of severe microcephaly. See the link below on how best to measure HC at birth.
Report if using a different definition or cut-off point (e.g. two standard deviations below the mean).
Cranial ultrasound, CT or MRI scans can confirm the diagnosis of an underlying brain abnormality.
Clinical and epidemiologic notes
Timing of measurement. Although head molding and/or swelling can occur during the birthing process, HC measurements should be obtained within the first 24 hours of life. Standards for HC are based on early measurements (i.e. INTERGROWTH-21st measurements were obtained before 12 hours of life and WHO measurements were obtained before 24 hours of life). Therefore, postponing HC measurements until after 24 hours of life to allow for birth process changes to subside results in not having appropriate comparison standards.
Calculating the percentile of HC. The INTERGROWTH-21st project provides data on HC based on a multicenter, multi-ethnic and population-based project. The online tool (http://intergrowth21.ndog.ox.ac.uk) can be used to enter data to calculate the percentile of the HC or to compare to standards based on the infant’s sex and gestational age (28).
Causes. Microcephaly is occasionally a normal trait in a family, so measuring parents’ HC if possible is reasonable. Many genetic syndromes are associated with microcephaly (> 1000 matches in www.OMIM.org) (29). Teratogenic conditions that can cause microcephaly include congenital rubella (P35.0), congenital cytomegalovirus (cCMV) (P35.1), congenital Zika (P35.4) and congenital toxoplasmosis (P37.1) infections. Detailed imaging and expert consultations (e.g. paediatric geneticist, paediatric neurologist) can help identify underlying causes of microcephaly.
Inclusions
Q02 Microcephaly
Exclusions
Small brain – without confirmation of HC measurement demonstrating microcephaly.
Microcephaly associated with anencephaly or encephalocele.
Acquired microcephaly; for example, secondary to a birth or delivery complication, postnatal insult or trauma, neonatal meningitis, and birth asphyxia.
Checklist for high-quality reporting
| Microcephaly – Documentation Checklist |
Describe in detail:
Report whether autopsy (pathology) findings are available and if so, report the results. |
Suggested data quality indicators
| Category | Suggested Practices and Quality Indicators |
| Description and documentation |
|
| Coding |
|
| Clinical classification |
|
| Prevalence |
|
Table of Contents
- Chapter 4: Diagnosing and Coding Congenital Anomalies
- 4.1 List of Selected External and Internal Congenital Anomalies to Consider for Monitoring
- 4.2 Congenital Malformations of the Nervous System: Neural Tube Defects
- 4.2a Anencephaly
- 4.2b Craniorachischisis (Q00.1)
- 4.2c Iniencephaly (Q00.2)
- 4.2d Encephalocele (Q01.0–Q01.83, Q01.9)
- 4.2e Spina Bifida (Q05.0–Q05.9)
- ›4.3 Congenital Anomalies of the Nervous System: Microcephaly
- 4.4 Congenital Malformations of the Ear
- 4.5a Overview Congenital Heart D: Prenatal Diagnosis and Postnatal Confirmation
- 4.5b Common Truncus (Q20.0)
- 4.5c Transposition of Great Arteries (Q20.3)
- 4.5d Tetralogy of Fallot
- 4.5e Pulmonary Valve Atresia (Q22.0)
- 4.5f Tricuspid Valve Atresia (Q22.4)
- 4.5g Hypoplastic Left Heart Syndrome (Q23.4)
- 4.5h Interrupted Aortic Arch (q25.21, Preferred; Also Q25.2, Q25.4)
- 4.6 Orofacial Clefts
- 4.7 Congenital Malformations of the Digestive System
- 4.8 Congenital Malformations of Genital Organs Hypospadias (Q54.0–Q54.9)
- 4.9a Congenital Malformations and Deformations of the Musculoskeletal System: Talipes Equinovarus (Q66.0)
- 4.9b Congenital Malformations and Deformations of the Musculoskeletal System: Limb Reduction Defects/Limb Deficiencies
- 4.9c Limb Deficiency Amelia (Q71.0, Q72.0, Q73.0)
- 4.9d Limb Deficiency: Transverse Terminal (Q71.2, Q71.3, Q71.30, Q72.2, Q72.3, Q72.30)
- 4.9e Limb Deficiency: Transverse Intercalary (Q71.1, Q72.1, Q72.4)
- 4.9f Limb Deficiency: Longitudinal Preaxial (Tibia, Radius, First Ray) (Q71.31, Q71.4, Q72.31, Q72.5)
- 4.9g Limb Deficiency: Longitudinal Postaxial (Fibula, Ulna, Fifth Ray) (Q71.30, Q71.5, Q72.30, Q72.6)
- 4.9h Limb Deficiency: Longitudinal Axial Limb Deficiency – Split Hand and Foot (Q71.6, Q72.7)
- 4.10 Abdominal Wall Defects
- 4.11 Chromosomal Abnormalities