3.13 Data Analysis
‹View Table of Contents
On this Page
Open for discussion, and then display the prevalence formula on screen. Discuss the difference between total prevalence, birth prevalence and live birth prevalence among participants (additional information can be found in Chapter 3 of WHO/CDC/ICBDSR Birth defects surveillance: a manual for programme managers (4)).
- Live birth prevalence of congenital anomalies = (live birth cases) / (total live births) × 10 000
- Birth prevalence of congenital anomalies = (live birth cases + fetal death (stillbirths) cases) / (total live births + fetal deaths (stillbirths)) × 10 000
- Total prevalence of congenital anomalies = (live birth cases + fetal death (stillbirths) cases + ETOPFA cases) / (total live births + total fetal deaths (stillbirths) + total ETOPFA) × 10 000
ETOPFA = elective termination of pregnancy for fetal anomaly
In a population-based surveillance programme, the total prevalence of congenital anomalies is calculated by aggregating the number of unduplicated cases (live births, stillbirths, and terminations) as the numerator, and the total number of live births and stillbirths in the source population as the denominator, for a specific catchment area and time period.
In hospital-based surveillance, the total prevalence of congenital anomalies is calculated by aggregating the number of unduplicated hospital cases (live births, stillbirths, and terminations) as the numerator, and the total number of live births and stillbirths from a participating hospital as the denominator, for a specific time period.
The prevalence of congenital anomalies is usually calculated and presented as prevalence per 10 000 births. Prevalence can be calculated for all congenital anomalies, specific individual congenital anomalies, or groups of anomalies. Prevalence cannot be calculated with only the number of cases – the numerator data – without having information about the denominator.
The following expression is used to calculate the birth prevalence of congenital anomalies, with the assumption that both live births and fetal deaths are being captured:
Birth prevalence = a/b × 10 000
- a = Number of live births and late fetal deaths (stillbirths) with a specific congenital anomaly (e.g. spina bifida) counted among the source population in a given year
- b = Number of live births and late fetal deaths (stillbirths) during the same year
The numerator includes live births and known fetal deaths (stillbirths) with congenital anomalies, and pregnancy terminations with congenital anomalies (if these data are available). The denominator comprises only live births and fetal deaths (stillbirths; if these data are available), because it is practically impossible to assess the total number of pregnancy losses. Because the number of pregnancy losses is relatively small, compared with the number of live births, their exclusion has little effect on the prevalence estimate. Spontaneous abortions (also called miscarriages) are not included in the numerator or in the denominator because it is practically impossible to assess the total number of spontaneous abortions. Note that terminations are not included in the denominator.
Possible responses:
- Hard to ascertain and usually not well reported in official demographic statistics
- Likely that the total number is small and, compared to the total number of births, their exclusion has little impact on the overall estimate
For more information on how to calculate total prevalence, live birth prevalence and birth prevalence, please refer to Chapter 3 of WHO/CDC/ICBDSR Birth defects surveillance: a manual for programme managers (4).
Activity 3.6
Ask participants to refer to Activity 3.6 in the Participant Workbook [457 KB, 21 Pages, Print Only]. Divide participants into groups. Ask participants to read the following casestudy and to calculate the prevalence of neural tube defects.
The United States National Birth Defects Prevention Network collects state-specific congenital anomalies surveillance data for annual publication of prevalence estimates and collaborative research projects. In 2010, data for 21 congenital anomalies from 2004–2006 were presented as national congenital anomalies prevalence estimates. The data presented in Table 3.1 are from population-based programmes that have different types of case ascertainment: active, hybrid and passive. Active ascertainment occurs when there is active review of multiple data sources to identify cases. Active ascertainment usually requires that the programme hires trained personnel to conduct abstraction from data sources. Passive ascertainment occurs when hospital staff report cases directly to the programme without verification of cases by the programme staff. An example of hybrid ascertainment is when hospital staff report cases and programme staff verify them.
| Number of Cases | ||||
| Neural tube defects | Active ascertainment (11 programmes)a |
Hybrid ascertainment (6 programmes)b |
Passive ascertainment (7 programmes)c |
National |
| Anencephaly | 697 | 211 | 192 | 1100 |
| Spina bifida | 1162 | 561 | 820 | 2543 |
| Encephalocele | 261 | 125 | 184 | 570 |
| Total neural tube defects | 2120 | 897 | 1196 | 4213 |
Source: Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE et al. Updated national birth prevalence estimates for selected congenital anomalies in the United States 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010; 88:1008–16. © 2010 by John Wiley & Sons, Inc. Reprinted by permission of John Wiley & Sons, Inc.
Data from programmes with active, hybrid or passive ascertainment.
a Number of live births in the active ascertainment programmes: 3 120 605.
b Number of live births in the hybrid ascertainment programmes: 2 075 973.
c Number of live births in the passive ascertainment programmes: 2 145 287.
Response
| Number of Cases | ||||
| Neural tube defects | Active ascertainment (11 programmes)a |
Hybrid ascertainment (6 programmes)b |
Passive ascertainment (7 programmes)c |
National |
| Anencephaly | 697 | 211 | 192 | 1100 |
| Spina bifida | 1162 | 561 | 820 | 2543 |
| Encephalocele | 261 | 125 | 184 | 570 |
| Total neural tube defects | 2120 | 897 | 1196 | 4213 |
Source: Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE et al. Updated national birth prevalence estimates for selected congenital anomalies in the United States 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010; 88:1008–16. © 2010 by John Wiley & Sons, Inc. Reprinted by permission of John Wiley & Sons, Inc.
Data are numbers of surveillance systems with active, hybrid or passive ascertainment.
a Number of live births in the active ascertainment programmes: 3 120 605.
b Number of live births in the hybrid ascertainment programmes: 2 075 973.
c Number of live births in the passive ascertainment programmes: 2 145 287.
Possible responses:
- The prevalence of specific defects varied by ascertainment method
- The prevalence of anencephaly varied considerably by ascertainment method
Possible reasons for differences include:
- Some programmes may include elective terminations
- Some programmes may include stillbirths in the numerator, denominator or both
- Some programmes may have conducted specialized prenatal ascertainment
- The prevalence of encephalocele was higher among the passive ascertainment method than among the hybrid or active ascertainment methods
Possible responses:
- Misclassification of cases at birth
- Reporting problems/congenital anomalies not reported – could say biased reporting, i.e. underreporting, overreporting or selective reporting
- Hybrid ascertainment methods are able to assess probable cases during followup and provide a definitive diagnosis
Trends
Trends in the context of congenital anomalies surveillance are:
- Used to provide information for needs assessments, programme planning, programme evaluation and policy development activities
- Used to generate tables and graphs for prevalences over relatively long periods of time
- Small numbers can introduce a large variation in yearly rates. When examining trends for small areas, small populations, or a narrow range of time, it may be necessary to combine several years of information.
Activity 3.7
Ask participants to refer to Activity 3.7 in the Participant Workbook. Divide participants into groups of 3 or 4 participants. Ask participants to read the scenario below.
In 1996, folic acid fortification of cereal grain products labelled as enriched became voluntary in the USA. In 1998, a mandate was passed requiring that these products be fortified with folic acid, to ensure an adequate supply of folate for women of childbearing age. The United States National Birth Defects Prevention Network collects information on neural tube defects by three major race/ethnic groups, and has data from the time period prior to mandatory folic acid fortification (1995–1997) and following the folic acid fortification mandate (1998–2010). The estimated annual prevalence of neural tube defects for nine hospitals in the USA during these time periods is presented in Table 3.3.
| Year | |||||||||||||
| Race/ ethnicity |
1995 | 1996 | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 |
| Hispanic | 9.20 | 10.84 | 9.69 | 7.37 | 7.83 | 6.45 | 6.63 | 6.98 | 6.95 | 6.63 | 6.27 | 5.69 | 6.04 |
| Black | 4.89 | 5.75 | 3.59 | 4.78 | 4.80 | 4.49 | 4.81 | 5.16 | 4.17 | 3.68 | 3.89 | 3.37 | 3.74 |
| Caucasian | 7.1 | 7.8 | 6.7 | 5.5 | 5.5 | 5.3 | 5.1 | 4.6 | 4.6 | 5.2 | 4.6 | 4.9 | 5.3 |
Source: CDC Grand Rounds: additional opportunities to prevent neural tube defects with folic acid fortification. MMWR Morb Mortal Wkly Rep. 2010;59(31):980–4.
Possible response:
- Folic acid fortification of staple foods is most likely responsible for the majority of the decline in prevalence of neural tube defects. The observable decline in the prevalence of neural tube defects is probably due to fortification.
Remember the following:
- Need for a meaningful scale on the Y axis, to improve understanding and the use of a timescale on the X axis to show trends
- Importance of axis labelling
- Use of highlighting effects or factors of interest, such as year in which fortification started in the USA (voluntary and mandatory)
- Need for clear and descriptive titles
Public health agencies have a long tradition of monitoring trends in rates of disease and death, and in medical, social and behavioural risk factors that may contribute to these adverse events. Trends in observed rates provide information for needs assessment, programme planning, programme evaluation, and policy development activities. Examining data over time also allows predictions to be made about future frequencies and rates of occurrence.
Typically in public health, trend data are presented as population-based rates. These data are accessed from large database systems such as national vital records, and show how rates change over relatively long periods of time, e.g. 10 years or more. Trend data can be visually presented through tables and graphs. Fig. 3.3 shows secular trend data for the prevalence of neural tube defects in the USA by race/ethnicity.
Fig. 3.3. Prevalence of neural tube defects (per 10 000 births) by race/ethnicity, USA, 1995–2007
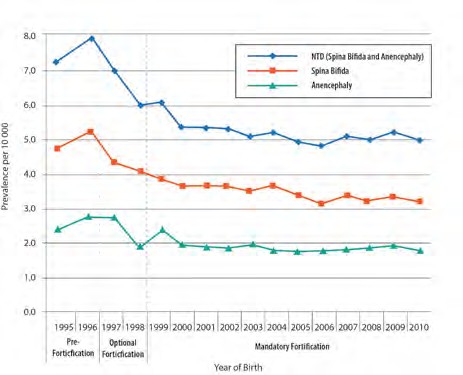
Source: National Birth Defects Prevention Network. Neural Tube Defect Ascertainment Project 2010 (http://www.nbdpn.org/docs/NTD_Fact_Sheet_11-13_for_website.pdf).
Possible response:
- There is a decline in the prevalence of neural tube defects.
Possible response:
- The change in prevalence started in the year 1997. After that point, the decrease in prevalence of neural tube defects accelerated through 2004, followed by a levelling off of the prevalence.
Possible responses:
- The introduction of folic acid fortification of staple foods
- The prevalence of neural tube defects was already declining and it is only a continuation of such decline, possibly due to other unmeasured factors
- Improved surveillance, more accurate data, fewer misclassifications
- Changes in ascertainment
Possible responses:
- Population changes due to migration
- Improved diagnostic procedures
- Enhanced reporting techniques
- Changes in the surveillance system or methods
- Changes in prevalence of other risk factors for the condition
- Changes in intervention
- World Health Organization. Congenital anomalies. Fact sheet No 370. October 2012 (http://www.who.int/mediacentre/factsheets/fs370/en/index.html , accessed 29 April 2015).
- Resolution WHA63.17. Birth defects. In: Sixty-third World Health Assembly, Geneva,17–21 May 2010. Geneva: World Health Organization; 2010 (http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_R17-en.pdf, accessed 29 April 2015).
- International statistical classification of diseases and related health problems, 10th revision. Geneva: World Health organization; 2015 (http://apps.who.int/classifications/icd10/browse/2015/en , accessed 24 February 2015).
- World Health Organization, National Center on Birth Defects and Developmental Disabilities from the United States Centers for Disease Control and Prevention (CDC), International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR). Birth defects surveillance: a manual for programme managers. Geneva: World Health Organization; 2014 (https://www.cdc.gov/ncbddd/birthdefectscount/documents/bd-surveillance-manual.pdf, accessed 10 February 2015).
- World Health Organization, National Center on Birth Defects and Developmental Disabilities from the United States Centers for Disease Control and Prevention(CDC), International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR). Birth defects surveillance: atlas of selected congenital anomalies. Geneva: World Health Organization; 2014 (http://apps.who.int/iris/bitstream/10665/127941/1/9789241564762_eng.pdf?ua=1 , accessed 10 February 2015).
- CDC Foundation. What is public health? (http://www.cdcfoundation.org/content/what-public-health , accessed 24 February 2015).
Table of Contents
- Module 3: Introduction to Surveillance Approaches
- 3.1 Epidemiology
- 3.2 Population Coverage
- 3.3 Case Ascertainment
- 3.4 Case-Finding
- 3.5 Case Inclusion
- 3.6 Inclusion Criteria
- 3.7 Inclusion of Pregnancy Outcomes
- 3.8 Description Formats for Congenital Anomalies
- 3.9 Core Ascertainment Variables
- 3.10 Data-Collection Methods and Tools
- 3.11 Data Collection/Management
- 3.12 Data-Management Protocol
- ›3.13 Data Analysis
- 3.14 Data Dissemination
- 3.15 Evaluation Questions 3