4.5a Congenital heart defects: Prenatal diagnosis and postnatal confirmation
‹View Table of Contents
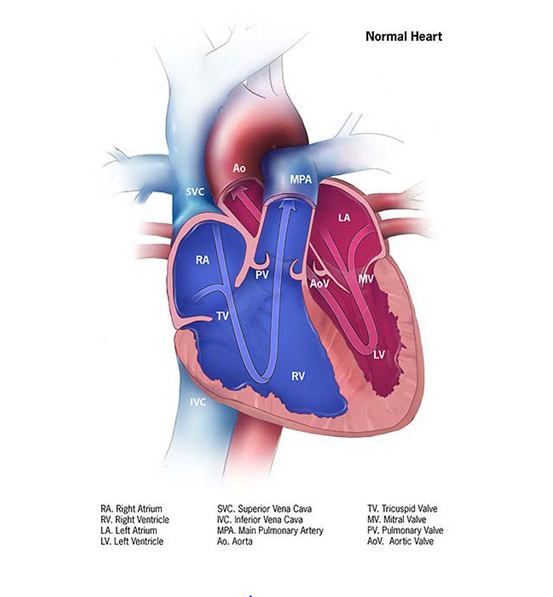
Fig. 4.14. Normal heart
Congenital heart defects/diseases (CHDs), and especially critical CHDs (the most severe CHDs that present with critical illness early in life), require early and accurate diagnosis for optimal care. Whereas newborn screening of critical CHDs via pulse oximetry is relatively straightforward and requires comparatively simple instrumentation, a precise diagnosis requires substantial clinical expertise and technologic means – ideally in the form of a paediatric cardiologist aided by imaging such as an echocardiogram. Because of the emphasis on early detection, evaluation of the fetal heart is increasingly a critical component of prenatal imaging. Current guidelines recommend specific cardiac views as part of the anatomic survey in obstetric sonograms. Findings indicating a possible CHD can be followed by more specialized imaging such as fetal echocardiography, to confirm and to better define the anomaly. The following sections briefly introduce some approaches to prenatal diagnosis and postnatal confirmation.
- Obstetric sonogram. Many obstetric guidelines for fetal ultrasound imaging include the four-chamber view (which focuses on the atria and ventricles) as well as outflow tracts views. The inclusion of these views improves the detection rate for many clinically severe CHDs such as d-transposition of the great arteries, which was often missed when only the four-chamber view was systematically performed.
- Fetal echocardiogram. The next step for an accurate and complete diagnosis is a fetal echocardiogram. This specialized imaging, typically supervised and read by a paediatric cardiologist, is often done when the obstetric sonogram detects a potential CHD, or in situations where the risk for CHDs in the fetus is higher, such as concerns about a syndrome or extracardiac anomalies, a known teratogenic exposure (e.g. retinoic acid), select maternal chronic illnesses (e.g. diabetes or uncontrolled phenylketonuria), or strong family history of CHDs. The fetal echocardiogram makes use of multiple views as well as color Doppler imaging to systematically scan the heart, segment by segment, to characterize the anatomy, rhythm and function of the fetal heart.
Prenatal detection
In high-risk pregnancies or when sonogram findings are suggestive of a CHD, obstetric sonograms followed by fetal echocardiogram might diagnose more than 80% of significant CHDs, especially critical CHDs.
- Detection rates for significant CHDs have improved over time but vary significantly by type of CHD. The conditions that are more easily diagnosed are critical CHDs that directly affect the ventricles – either the left ventricle (e.g. hypoplastic left heart syndrome or critical aortic stenosis) or the right ventricle (e.g. tricuspid atresia or pulmonary atresia with intact ventricular septum). Conditions that might be missed – if, for example, the outflow tract view is not included or is inadequate in an obstetric sonogram – are those that primarily involve the outflow tracts but impact the ventricles subtly, if at all (e.g. simple d-transposition of the great arteries).
- Detection of CHDs also likely depends on the operator (knowledge, skill and technique), the imaging equipment, time allotted for obstetric scan, gestational age, maternal body habitus and fetal lie. Additionally, there are system-level factors that might influence access to and use of prenatal services, including socioeconomic status, insurance status, education and location (e.g. rural versus urban).
Postnatal confirmation of prenatal diagnoses
These considerations can provide some guidance to surveillance systems that collect data on prenatal diagnosis. In general, prenatal diagnoses should be confirmed postnatally, typically by echocardiogram. This approach is expected to provide the most accurate and complete diagnosis, establish prevalence and allow for longitudinal outcome studies. If a prenatal diagnosis is not followed by postnatal confirmation (e.g. because of termination of pregnancy, loss of follow-up, early neonatal death, comfort care without imaging, etc.), then a reasonable approach for a programme is as follows:
- Develop an explicit and transparent process to determine whether a case is confirmed or probable. The parameters might vary depending on the type of CHD and the local context (e.g. experience, training of the operator, correlation between pre- and postnatal diagnoses in other cases, etc.).
- Have a field in the database associated with each diagnosis that specifies whether the diagnosis was a prenatal diagnosis only versus confirmed after birth. This allows for a flexible and easy parsing of cases for different types of analyses and comparisons (internally as well as with other programmes).
Postnatal screening and diagnoses
In hospitals where there is a neonatologist or a paediatrician it would be possible to suspect CHD based on clinical signs and confirm with expert consultation and imaging. Newborn screening for critical CHDs using pulse oximetry provides a non-invasive and relatively simple way to screen for severe cardiac disease very early in life, before clinical deterioration becomes obvious and before the infant is discharged home. Screening detects low oxygen saturation but does not provide a specific diagnosis (the infant might have a CHD or other serious disease such as pneumonia or sepsis). Expert consultation and imaging (typically an echocardiogram) can quickly provide a firm and specific diagnosis, and this information can be used to guide targeted management and care for better outcomes.
Table of Contents
- Chapter 4: Diagnosing and Coding Congenital Anomalies
- 4.1 List of Selected External and Internal Congenital Anomalies to Consider for Monitoring
- 4.2 Congenital Malformations of the Nervous System: Neural Tube Defects
- 4.2a Anencephaly
- 4.2b Craniorachischisis (Q00.1)
- 4.2c Iniencephaly (Q00.2)
- 4.2d Encephalocele (Q01.0–Q01.83, Q01.9)
- 4.2e Spina Bifida (Q05.0–Q05.9)
- 4.3 Congenital Anomalies of the Nervous System: Microcephaly
- 4.4 Congenital Malformations of the Ear
- ›4.5a Overview Congenital Heart D: Prenatal Diagnosis and Postnatal Confirmation
- 4.5b Common Truncus (Q20.0)
- 4.5c Transposition of Great Arteries (Q20.3)
- 4.5d Tetralogy of Fallot
- 4.5e Pulmonary Valve Atresia (Q22.0)
- 4.5f Tricuspid Valve Atresia (Q22.4)
- 4.5g Hypoplastic Left Heart Syndrome (Q23.4)
- 4.5h Interrupted Aortic Arch (q25.21, Preferred; Also Q25.2, Q25.4)
- 4.6 Orofacial Clefts
- 4.7 Congenital Malformations of the Digestive System
- 4.8 Congenital Malformations of Genital Organs Hypospadias (Q54.0–Q54.9)
- 4.9a Congenital Malformations and Deformations of the Musculoskeletal System: Talipes Equinovarus (Q66.0)
- 4.9b Congenital Malformations and Deformations of the Musculoskeletal System: Limb Reduction Defects/Limb Deficiencies
- 4.9c Limb Deficiency Amelia (Q71.0, Q72.0, Q73.0)
- 4.9d Limb Deficiency: Transverse Terminal (Q71.2, Q71.3, Q71.30, Q72.2, Q72.3, Q72.30)
- 4.9e Limb Deficiency: Transverse Intercalary (Q71.1, Q72.1, Q72.4)
- 4.9f Limb Deficiency: Longitudinal Preaxial (Tibia, Radius, First Ray) (Q71.31, Q71.4, Q72.31, Q72.5)
- 4.9g Limb Deficiency: Longitudinal Postaxial (Fibula, Ulna, Fifth Ray) (Q71.30, Q71.5, Q72.30, Q72.6)
- 4.9h Limb Deficiency: Longitudinal Axial Limb Deficiency – Split Hand and Foot (Q71.6, Q72.7)
- 4.10 Abdominal Wall Defects
- 4.11 Chromosomal Abnormalities