2018 Salmonella Infections Linked to Raw Chicken Products – Antibiotic Resistance Information
Interpretation
- Most isolates tested are resistant to ampicillin, ceftriaxone, and ciprofloxacin, and some isolates are resistant to trimethoprim-sulfamethoxazole, which are antibiotics used to treat Salmonella infections.
- The resistance profiles of isolates in this outbreak might affect treatment choices. Although most people with Salmonella infection recover without antibiotic treatment, people at risk of complications and those with severe illness may require antibiotics. Get advice for clinicians.
- Resistant bacteria might be more likely than susceptible ones to cause bloodstream infections or prolonged illness.
- The presence of any resistance is a concern because the genes that confer resistance may spread to other bacteria in people, animals, or the environment.
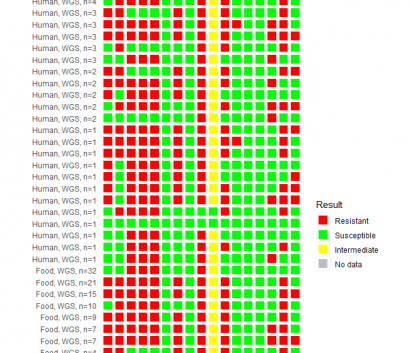
Results of Antimicrobial Susceptibility Testing and Whole Genome Sequencing
CDC uses two categories of testing to determine antibiotic resistance of bacteria: antimicrobial susceptibility testing (AST) and analysis of whole genome sequencing (WGS).
Results are summarized in the figure below.
- Results are available for 240 isolates from 97 ill people and 143 food samples
- Resistance was predicted by WGS for 234 isolates and confirmed for 6 isolates by AST
- Isolates were resistant to ampicillin (58.3%), ceftriaxone (57.9%), chloramphenicol (62.5%), ciprofloxacin (97.1%), fosfomycin (22.1%), gentamicin (66.7%), hygromycin (64.2%), kanamycin (25.8%), nalidixic acid (97.1%), streptomycin (92.5%), sulfisoxazole (91.3%), tetracycline (91.3%), and trimethoprim-sulfamethoxazole (2.9%).
Antibiotic Resistance for Isolates from Outbreak of Salmonella Infantis Infections Linked to Raw Chicken Products, as of February 19, 2019
Detailed methods and antibiotics tested can be found on the Methods for Testing Bacteria for Resistance to Antibiotics page.
Some drugs may not be appropriate to treat certain infections; in vitro susceptibility may not predict clinical effectiveness; and interpretations of susceptible or resistant may only apply to certain types of bacteria. More information is available at CLSI (CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2020.)
When resistance results are available from both AST and WGS, only AST results are provided for those drugs on the panel. For drugs not on the panel, results predicted by WGS are shown when available. Each row percentage represents mutually exclusive combinations of specimen source, testing method, and resistance pattern.